Table 7.
Analysis of substances from the NICEATM set that were predicted incorrectly by all three in silico models, the justification for the TIMES-SS and Derek predictions are given, VEGA does not give a mechanistic description. The authors’ proposed rationales for the incorrect predictions are also given. Mechanistic domains – Michael Acceptor (MA), Schiff Base former (SB), Acyl transfer agent, SN2 etc. make reference to the reaction pathways described in Aptula and Roberts [33]
| Structure | DSSTox Substance Identifier (DTXSID) |
CAS | Experimental sensitisation outcome |
TIMES-SS | Derek Nexus |
Author proposed rationales/comments |
|---|---|---|---|---|---|---|
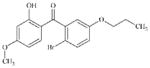
|
DTXSID60697617 | 190965-45-8 | LLNA-NS | Aromatic carbonyl compounds | Resorcinol or precursor | SB would be more plausible if COAr was ortho to OH was an aldehyde. Resorcinol formation is deactivated by the COAr which is electron withdrawing and therefore deactivated resorcinol’s oxidation. |
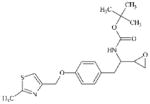
|
DTXSID90469546 | 313680-92-1 | LLNA-NS | Epoxides, Aziridines and Sulfuranes | Epoxide | Epoxide is probably insufficiently reaction (see [36]). |
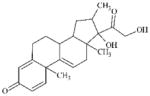
|
13504-15-9 | LLNA-NS | alpha, beta-Carbonyl compounds with polarised double bonds | 1,2-Dicarbonyl compound or precursor | This metabolic activation is probably not significant in mouse skin vs guinea pig or man (see 50-24-8). | |
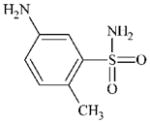
|
DTXSID40284367 | 6973-09-7 | LLNA-NS | Metabolite: Quinone methide(s)/imines, Quinoide oxime structure, Nitroquinones, | Aromatic primary or secondary amine | Oxidation is deactivated by SO2Me. |
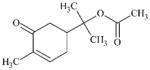
|
DTXSID60337127 | 87578-93-6 | LLNA-NS | alpha, beta-Carbonyl compounds with polarised double bonds | alpha, beta-Unsaturated ketone or precursor | Low reactivity as a methacrylate. |
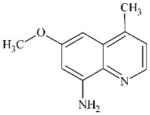
|
DTXSID70345475 | 57514-21-3 | LLNA-NS | Metabolites: Hydroperoxides | Aromatic primary or secondary amine | Oxidation of the aromatic amine would be deactivated by OMe and the fused pyridine ring. |
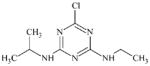
|
DTXSID9020112 | 1912-24-9 | LLNA-NS | Activated aryl and heteroaryl compounds | Activated N-heterocycle | The negative experimental outcome is surprising. Would expect this substance to be reactive as a SNAr. Hydrolysis might account for the NS outcome reported. |
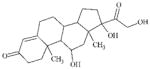
|
DTXSID9020112 | 50-23-7 | LLNA-NS | Ketones | 1,2-Dicarbonyl compound or precursor | Metabolic activation in mouse skin is probably not significant to lead to a sensitising outcome. (see 50-24-8). |
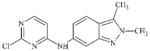
|
DTXSID50469840 | 444731-74-2 | LLNA-NS | Activated aryl and heteroaryl compounds | Activated N-heterocycle | Surprising outcome. Would expect this substance to react as a SNAr electrophile, hydrolysis could be a competing factor. |
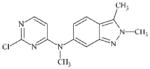
|
DTXSID70469403 | 444731-75-3 | LLNA-NS | Activated aryl and heteroaryl compounds | Activated N-heterocycle | Surprising outcome. Would expect this substance to react as a SNAr electrophile, hydrolysis could be a competing factor. |
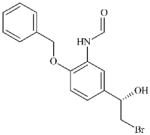
|
DTXSID40456769 | 201677-59-0 | LLNA-NS | Epoxides, Aziridines and Sulfuranes | Alkyl halide | OH relative to the Br leaving group has a deactivating effect. Not sufficiently reactive to act as a SN2 electrophile. |
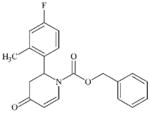
|
DTXSID10620233 | 414909-98-1 | LLNA-NS | Carbamates | alpha, beta-Unsaturated ketone or precursor | Poor acyl transfer agent, weak SN2 and deactivated as MA by beta Nitrogen. |
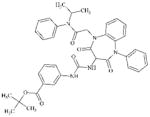
|
DTXSID70725319 | 305366-94-3 | LLNA-S | No Alerts | No Alerts | Right hand side benzene ring has 2 ortho N substituents. Hydrolysis of the amides in the 7 membered ring would give rise to a ortho-di(aryl)amino benzene which is oxidisable to a quinone imine that could act as a MA. |
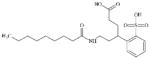
|
168151-92-6 | LLNA-S | No Alerts | No Alerts | Anionic with surfactant features, likely false positive (FP) result. | |
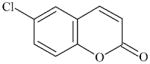
|
2051-59-4 | LLNA-S | No Alerts | No Alerts | MA – lactone; phenyl ester. | |
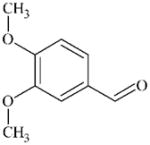
|
DTXSID7026285 | 120-14-9 | LLNA-S | No Alerts | No Alerts | Aromatic aldehyde SB former that happens to be sufficiently reactive to sensitise. |
