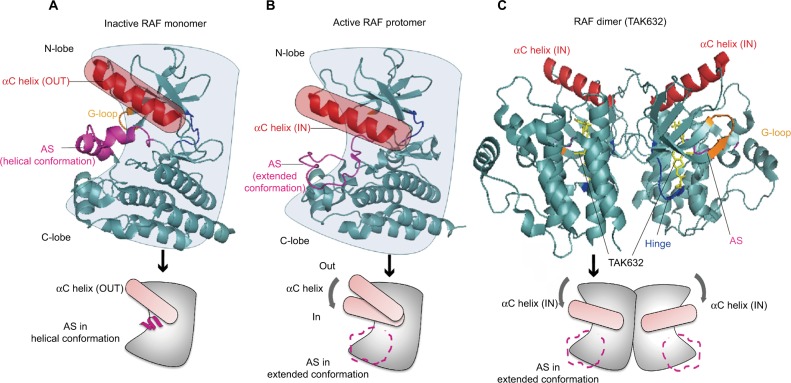
Figure 1.
Insight on the structural characteristics of RAF activation.
Notes: (A) The inactive RAF is a monomer, and the kinase domain has the characteristic N-lobe and C-lobe linked by a hinge. The positioning of the αC helix of N-lobe maintains its “OUT” conformation, and the AS forms as a helical conformation (PDB ID: 4RZV). (B) After the RAF activation, the conformation of AS is extended, which induces the αC helix to move inward to the “IN” position. The active RAF displays a comprehensive static “closed” conformation (PDB ID: 4MNE). (C) The structural characteristics of typical RAF inhibitors (TKA632) bound to a BRAF dimer show that both αC helixes are in the IN position (PDB ID: 4KSP).
Abbreviations: AS, activation segment; PDB, Protein Data Bank.