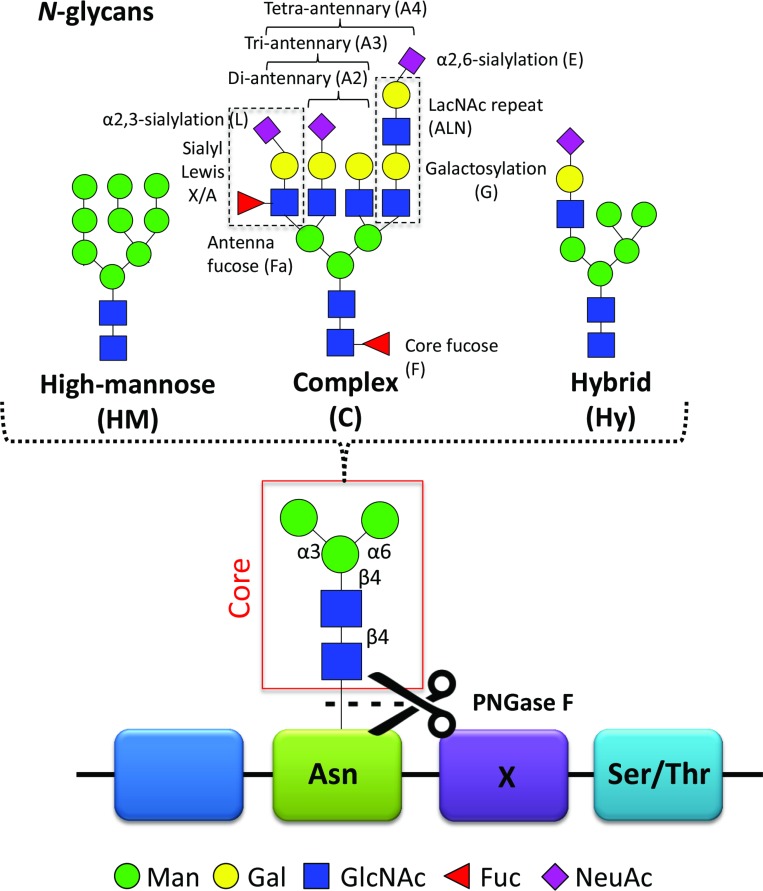
Figure 1. N-glycans.
N-Glycans are covalently linked to an asparagine (Asn) of the peptide backbone. The consensus sequence Asn-X-Ser/Thr (Ser = serine, Thr = threonine), where X can be any amino acid except proline, forms a potential N-glycosylation site. Importantly, not all potential N-glycosylation sites are occupied by N-glycans. The enzyme peptide N-glycosidase F (PNGase F) can cleave the N-glycan off the asparagine. All N-glycans consist of a common core-structure of three mannoses (Man) and two N-acetylglucosamines (GlcNAc): Man3GlcNAc2. The core can be further elongated by i) only mannoses resulting in high-mannose type N-glycans; ii) antennae containing galactose (Gal) and GlcNAc repeats, referred to as LacNAc repeat, antenna- or core-attached fucoses (Fuc) and terminal sialic acids (N-acetylneuraminic acid, NeuAc) forming complex type glycans. Depending on the number of antennae added, one distinguishes between di-, tri- and tetra-antennary N-glycans; iii) a mixture of the two with one high-mannose arm and one complex-type arm forming the hybrid type N-glycan. The illustrated cartoons represent examples, combinations, linkages and the overall extend of the elongations is highly heterogeneous.