Figure 1. Schematic illustrations of typical salt concentrations in mammalian environments and anions and cations being exchanged with a saline buffer in the region around a biomolecule.
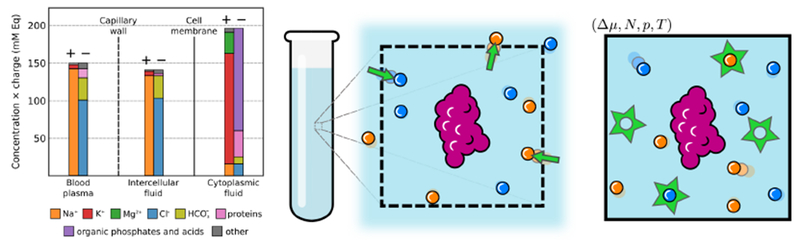
Left: The ion compositions of intra- and intercellular mammalian environments are shown as millimolar equivalents (mM Eq), which is the ion concentration multiplied by the absolute charge of the ion. The primary contribution to the ionic-strength are monovalent ions (Na+, K+, Cl−), divalent cations (predominantly Mg2+), complex salt and buffer molecules, and charged proteins. In addition to the significant difference between the ionic composition of the cytoplasmic fluid and extracellular fluid, organelles can also have markedly different ionic concentrations to the cytoplasmic fluid1. Over large lengthscales, environments are approximately electrostatically neutral; electrostatic potentials across cell membranes are maintained by an imbalance of anions and cations that is minuscule relative to the total number ions2. Figure adapted from2 and3. Middle: In a very large system, where the number of water molecules and number of ions are fixed, significant fluctuations can occur in the ionic strength of the local environment of a biomolecule (in purple). The local environment is represented by a dashed line, within which the number of water molecules and ions fluctuate at equilibrium. Right: A simulation with an osmostat replicates the natural variations in ionic strength around a biomolecule that would occur if the system were embedded in an infinite saline reservoir at a fixed macroscopic salt concentration. Anions and cations (blue and orange spheres) are inserted and deleted (green stars) from the system using semigrand canonical Monte Carlo moves that exchange explicit water molecules for the ions in a manner that maintains total charge neutrality. The reservoir is completely defined by its thermodynamic parameters, which in this case include the difference in the chemical potential for two water molecules and NaCl, Δμ (= Δμ2·H2O–NaCl), pressure p, and temperature, T.
