Figure 3. Schematic illustration of the nonequilibrium candidate Monte Carlo (NCMC) alchemical protocol used to insert NaCl.
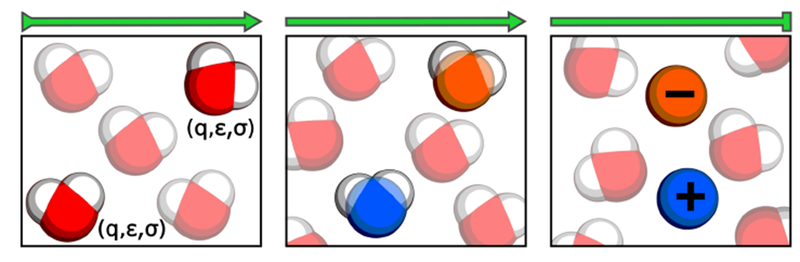
Two water molecules are chosen at random for transformation into Na+ (blue sphere) and Cl− (orange sphere). Over a number of NCMC steps, the nonbonded parameters of each atom in the water molecules, namely the partial charges, q, Lennard-Jones energy well depths, ϵ, and Lennard-Jones separation parameters, σ, are transformed into the nonbonded parameters of the ions along a linear interpolation of the parameters. The hydrogen atoms and extra charge sites (if present) of the water model remain attached to the ions as non-interacting dummy atoms. The entire NCMC proposal is then accepted or rejected according to the probability given in equation 56. Note that osmostat NCMC moves are mixed with standard Langevin integration at a fixed timestep to obtain fully ergodic sampling. A full description of the Monte Carlo and NCMC procedure used here is provided in Appendix 3.
