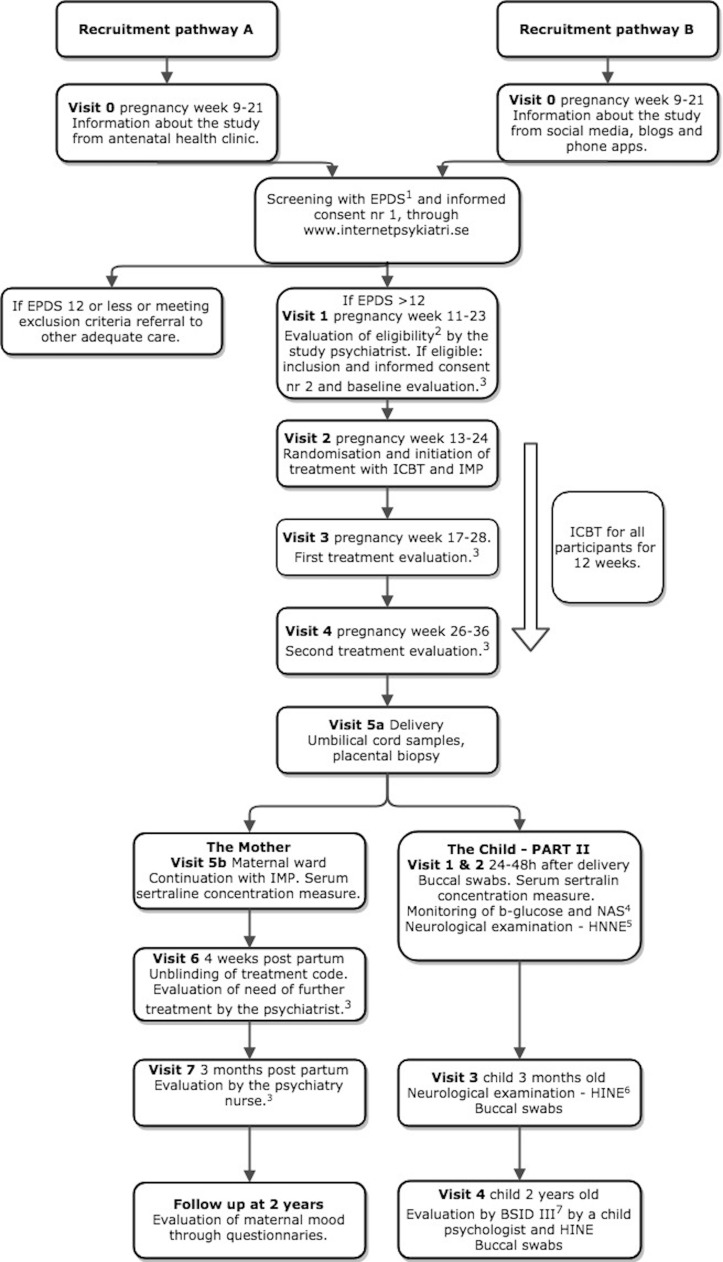
Figure 1.
Trial design and participant timeline for the two recruitment pathways. The figure also shows the treatment with placebo/sertraline, the investigational medical product (IMP) from visit 2 (pregnancy weeks 13–24) to visit 6 (4 weeks postpartum) and the internet-based cognitive-behavioural therapy (ICBT) for both groups for 12 weeks, between visit 2 and visit 4 (pregnancy weeks 26–36) and monitoring of therapy for the two groups. The postpartum follow-up of both mother and child are shown. The different scales and examinations are as presented in the figure: (1) Edinburgh Postnatal Depression Scale (EPDS),41 (2) diagnosis of moderate depression is confirmed according to clinical standard evaluation. Inclusion and exclusion criteria presented in table 1. (3) Evaluation with Montgomery-Åsberg Depression Scale (MADRS),64 (4) Modified Finnegan Neonatal Abstinence Scale (NAS),58 (5) Hammersmith Neonatal Neurological Examination (HNNE),57 (6) Hammersmith Infant Neurological Examination (HINE)65 and (7) Bayley Scales of Infant and Toddler Development III (BSID III).48