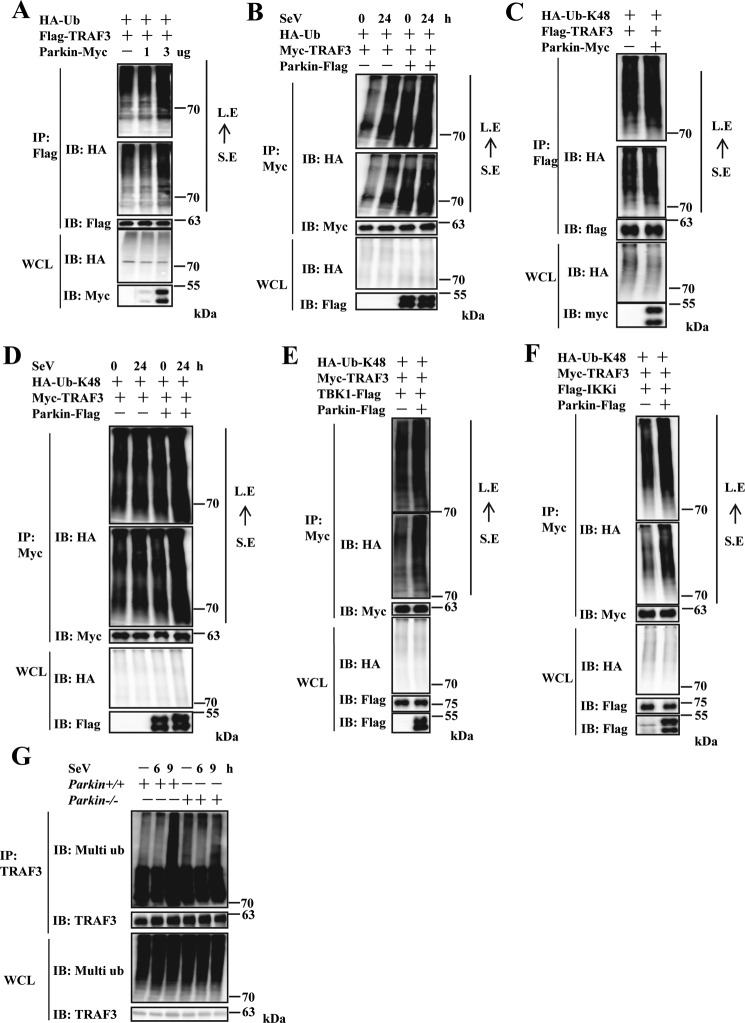
Figure 6.
Parkin increases Lys48-linked ubiquitination of TRAF3. A, overexpression of Parkin enhanced exogenous TRAF3 polyubiquitination in a dose-dependent manner. HEK293 cells were transfected with Flag–TRAF3 and HA–Ub together with empty vector or increased dose of Parkin–Myc plasmids. 24 h after transfection, the cells were treated with MG132 (final concentration, 25 μm) for 4 h. The cell lysates were immunoprecipitated with anti-Flag beads and analyzed by immunoblotting (IB) with the indicated antibodies. Bottom panel, expression of exogenous proteins in WCL. B, overexpression of Parkin increased exogenous TRAF3 polyubiquitination in the presence of SeV infection. HEK293 cells were transfected with Myc-TRAF3 and HA–Ub together with empty vector or Parkin–Flag plasmids. 24 h after transfection, the cells were infected with SeV for 24 h and then treated with MG132 (final concentration, 25 μm) for 4 h. The cell lysates were immunoprecipitated with anti-Myc beads and analyzed by immunoblotting with the indicated antibodies. Bottom panel, expression of exogenous proteins in WCL. C, Parkin overexpression increased Lys48-linked ubiquitination of exogenous TRAF3. HEK293 cells were transfected with Flag–TRAF3 and HA–Ub–Lys48 together with empty vector or Parkin–Myc plasmids. 24 h after transfection, the cells were treated with MG132 (final concentration, 25 μm) for 4 h. The cell lysates were immunoprecipitated with anti-Flag beads and analyzed by immunoblotting with the indicated antibodies. Bottom panel, expression of exogenous proteins in WCL. D, Parkin overexpression enhanced Lys48-linked ubiquitination of exogenous TRAF3 in the presence of SeV infection. HEK293 cells were transfected with Myc-TRAF3 and HA–Ub–Lys48 together with empty vector or Parkin–Flag plasmids. 24 h after transfection, the cells were infected with SeV for 24 h and then treated with MG132 (final concentration, 25 μm) for 4 h. The cell lysates were immunoprecipitated with anti-Myc beads and analyzed by immunoblotting with the indicated antibodies. Bottom panel, expression of exogenous proteins in WCL. E and F, Parkin overexpression increased Lys48-linked ubiquitination of exogenous TRAF3 with co-expression of IKKi or TBK1. HEK293 cells were transfected with Myc-TRAF3, HA–Ub–Lys48, and TBK1–Flag (E) or Flag–IKKi (F) together with empty vector or Parkin–Flag plasmids. 24 h after transfection, the cells were treated with MG132 (final concentration, 25 μm) for 4 h. The cell lysates were immunoprecipitated with anti-Myc beads and analyzed by immunoblotting with the indicated antibodies. Bottom panel of A, B, C, D, E, or F, respectively, expression of exogenous proteins in WCL. G, Parkin deficiency significantly reduced endogenous TRAF3 ubiquitination induced by SeV infection in Parkin−/− immortalized MEFs. Immortalized Parkin+/+ and Parkin−/− MEFs were left untreated or infected with SeV for the indicated times, then treated with MG132 (final concentration, 25 μm) for 4 h. The cell lysates were immunoprecipitated with anti-TRAF3 antibody and analyzed by immunoblotting with the indicated antibodies. Bottom panel, expression of endogenous proteins in WCL. S. E, short exposure; L. E, long exposure.