Abstract
BACKGROUND AND OBJECTIVES
We aimed to evaluate long-term treatment outcomes and toxicity profile of combined radiotherapy and chemotherapy in Saudi women with locally advanced cervical cancer.
DESIGN AND SETTINGS
Retrospective study in a tertiary care cancer center in Riyadh, Saudi Arabia.
METHODS AND MATERIALS
The medical records of patients with histopathologically proven, locally advanced cervical cancer were analyzed. These patients received three-dimensional conformal radiotherapy with concurrent chemotherapy followed by high dose rate brachytherapy in our center between July 2007 and April 2012. The data regarding the safety profile, response rates, occurrence of locoregional or distant failure, disease-free survival, and overall survival rates were recorded.
RESULTS
The median follow-up period was 60 months (range, 8–66) for 74 patients. The median age of study population was 52.3 years (32–78), and the stage IIB was the predominant stage (49 patients [66.2%]). A total of 45 patients (60.9%) had radiologic-positive pelvic ± para-aortic lymphadenopathy. The 5-year locoregional and distant control rates were 84.3% and 78.5%, respectively. The 5-year disease-free and overall survival rates were 75.7% and 64.5%, respectively. Stage, nodal status, and hemoglobin levels were found to be important prognostic factors for locoregional and distant control. Acute grade 3 hematological and nonhematological toxicities were seen in 4 (5.4%) and 4 (5.4%) patients. Late toxicities were mild, and only 1 (1.4%) patient presented with subacute intestinal obstruction.
CONCLUSION
Concurrent chemoradiation in Saudi women with locally advanced cervical cancer showed better locoregional and distant control and survival rates with minimal toxicity.
Cervical cancer ranks as the 11th most frequent cancer among women in Saudi Arabia, and the 8th most frequent cancer among women in the age group of 15 to −44 years, and every year 152 new cases are diagnosed, of whom 55 (36.1%) die from the disease.1 Because of inadequate cytological screening program and poor acceptance by women population, the majority of women are diagnosed at locally advanced stages—International Federation of Gynecologists and Obstetrics (FIGO) stage IIB–IVA—for which surgery is inadequate due to parametrial invasion.2 The standard treatment for locally advanced cervical cancer is prescribed as combined chemotherapy and pelvic irradiation, based on the results of 5 Western and North American randomized trials showing survival benefit by 10% to 15% and local and distant recurrence reduction rates by 30% to 40%.3–5 However, disease-free survival (DFS) and overall survival (OS) benefit of combined chemotherapy and pelvic irradiation in Saudi women with locally advanced cervical cancer is not well known. El-Senoussi, et al have reported 5-year DFS and OS rates of 68.3% and 57.9%, respectively, in 164 Saudi women with FIGO stage I and II cervical cancer.6 However, this study incorporated conventional EBRT followed by low dose rate brachytherapy techniques. However, over the last decade the advent of novel radiation therapy techniques (three-dimensional conformal radiation therapy [3DCRT] and intensity modulated radiation therapy [IMRT]) have shown improvements in locoregional control and minimal toxicity.
We aimed to evaluate the efficacy, toxicity, and treatment outcomes of 3DCRT pelvic irradiation with chemotherapy followed by high dose rate brachytherapy in Saudi women with locally advanced cervical cancer and also to evaluate the prognostic factors affecting locoregional and distant control.
METHODS AND MATERIALS
Eligibility
After getting approval from institutional Review Committee, the medical records of patients with locally advanced cervical cancers were looked for demographic, symptomatology, tumor characteristics, radiologic imaging, pathologic examination, radiation therapy techniques, concurrent chemotherapy regimen, acute and chronic side effects, and response rates. Patients with locally advanced cervical cancer between July 2007 and May 2012 were studied when they met the following eligibility criteria:
(1) histologically proven squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma of cervix uteri; (2) clinical and radiologic FIGO stage IIB–IVA with no other evidence for distant metastasis outside the pelvis; (3) Eastern Co-operative Oncology Group performance status 0 to 2; and (4) patients who received radical concurrent chemoradiation. Patients with a previous history of hysterectomy, retroperitoneal surgery, abdominal or pelvic radiotherapy, prior chemotherapy, and pregnancy were excluded.
Treatment Protocol
External beam irradiation
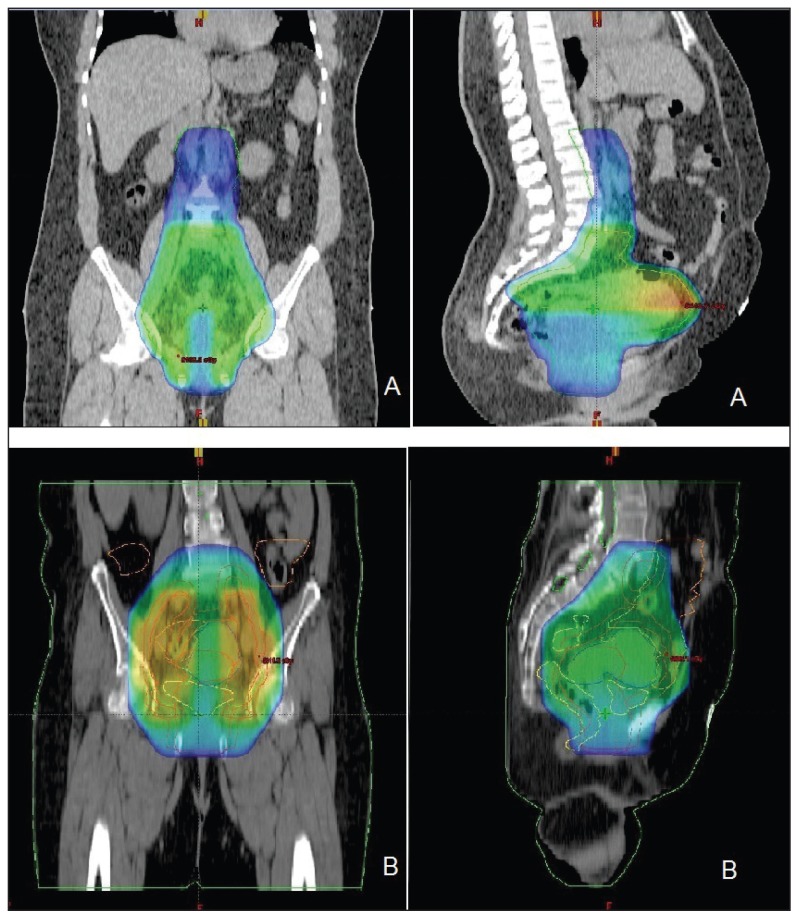
All patients were scanned for simulation on a CT simulator from epigastrium to mid thighs, and 3DCRT planning was performed. After data acquisition, the gross tumor volume (GTV; clinical target volume [CTV-1] (GTV+uterus+1 cm margins+common, internal, and external iliac lymph nodes±para-aortic nodes), planning target volume (PTV-1; CTV-1 + 0.5 to 1 cm margins), CTV-2 (parametrium + 1 cm margin with 4 cm midline shielding), PTV-2 (CTV+ 0.5 cm margins), and organs at risks (kidneys, small bowel, bladder, rectum, and femoral heads) were delineated. Four equally spaced, coplanar 3DCRT field plans for pelvis and anteroposterior and posteroanterior field for para-aortic regions were generated. The prescribed radiation doses were 45 to 50.4 Gy/25 to 28 fractions to PTV-1 and 54 to 59.4Gy/30 to 33 fractions to PTV-2, 5 days per week, and up to 7% variation was considered acceptable. During planning, the mean dose to the rectum was constrained to <50 Gy, and the total doses to the small bowel, kidneys and bladder were constrained to <45 Gy, <20 Gy, <60 Gy, respectively (Figures 1 and 2).
Figure 1.
Initial phase radiotherapy (A) extended field and (B) pelvic field three-dimensional conformal radiation therapy (3DCRT) plans showing 45 Gy to PTV-1 in coronal and sagittal views.
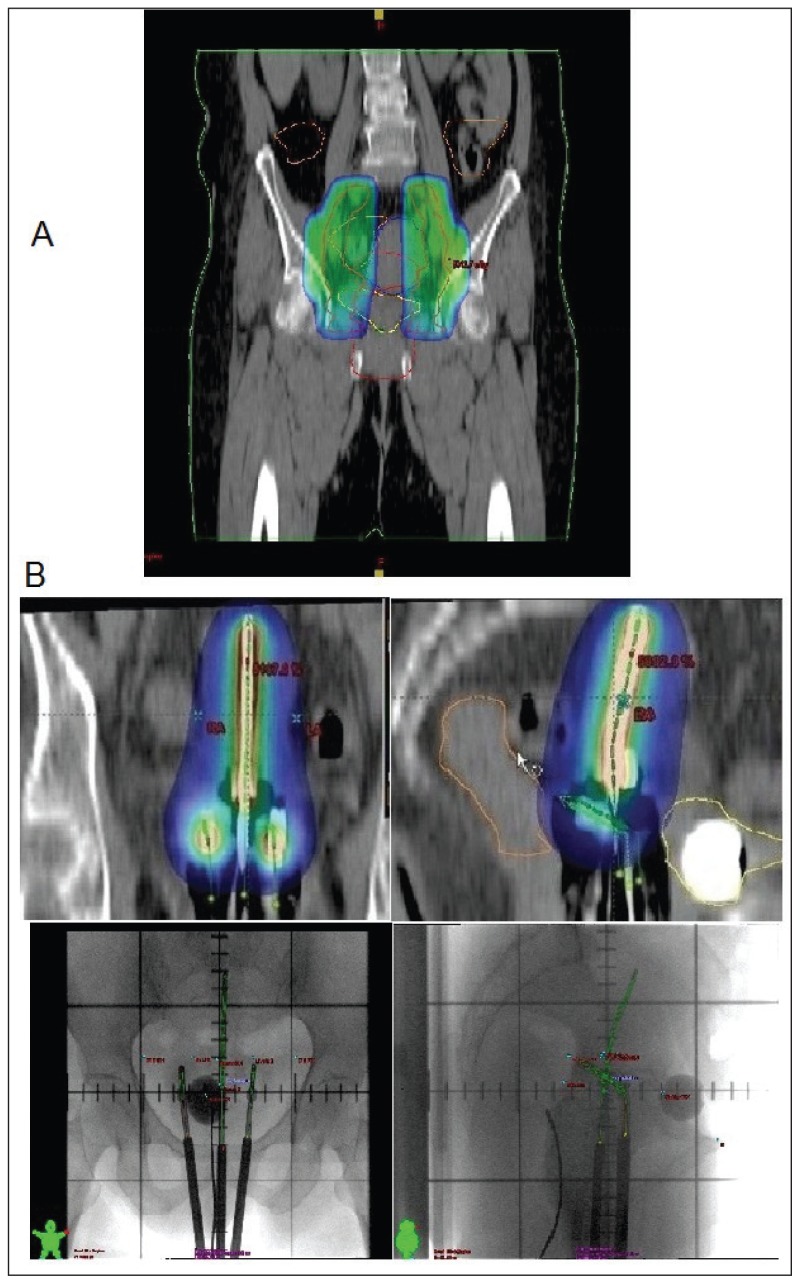
Figure 2.
(A) Parametrial boost of 9 Gy with midline shielding followed by high dose rate brachytherapy 21 Gy in 3 sessions.
Concurrent chemotherapy
All patients received weekly cisplatin 40 mg/m2 before the administration of radiotherapy for 6 doses. During concurrent chemoradiation (CCRT), the doses modifications were done on weekly basis. If the WBC count was below 2000/mm3 or the platelet count was below 50 000/mm3, all chemotherapy for that week was omitted. If the white blood cell count was below 1000/mm3 or the platelet count was below 25 000/mm3, CCRT was withheld until the white blood cell count and the platelet count recovered to 1000/mm3 or greater and 25 000/mm3 or greater, respectively. If the serum creatinine level was above 1.8 mg/dL, the cisplatin was withheld. Patients were deemed unsuitable for further chemotherapy if the delay to resume treatment was longer than 2 weeks.
High dose rate brachytherapy
Fletcher-Suit tandem and ovoids afterloading applicators were used for high dose rate (HDR)-brachytherapy with iridium-192 sources once a week under conscious sedation after 45 Gy of external beam irradiation therapy (EBRT). A dose of 7 Gy per fraction using 4–6 insertions to point A with a total dose of 21 Gy was delivered, based on the dose limit derived from the treatment plan for the rectum and bladder. The dose constraints were 75 Gy and 80 Gy for the rectum and bladder, respectively (Figure 2).
Toxicity scoring
The National Cancer Institute Common Toxicity Criteria version 2.0 were used to score acute radiation and chemotherapy toxicity (<90 days from the start of radiation therapy). During CCRT, weekly weight, performance status, pelvic examination findings, hematologic, and blood chemistry determinations were also recorded.
The Radiation Therapy Oncology Group Late Radiation Morbidity Scoring Criteria were used to score radiation toxicity persisting beyond 90 days from the completion of radiotherapy. After completion of the CCRT, patients were seen every 3 months for the first 2 years, and every 6 months thereafter at radiation oncology and gynecology oncology clinics. The response evaluation consisted of a physical and pelvic examination; a Pap smear; hematology, hepatic, renal function tests; computed tomography (CT) chest and abdomen; and pelvic magnetic resonance imaging (MRI) every 6 months for the first 2 years.
Statistical analysis
The primary endpoints were toxicity, locoregional and systemic control, and DFS and OS rates. DFS was defined as the duration between the completion of CCRT and the date of documented disease recurrence, death resulting from the cancer, and/or last follow-up visit (censored). OS was defined as the duration between the completion of CCRT and the date of patient death or last follow-up visit (censored). The probabilities of local, para-aortic, and distant control, and DFS and OS rates were determined with the Kaplan-Meier method. The comparisons for various endpoints were performed using the log-rank test. The Student unpaired t test was used to determine the significance of the difference between the 2 groups. A P value of .05 was considered statistically significant. Cox regression model was used to evaluate the effect of the potential prognostic factors on locoregiona controll, distant control, and DFS. Bonferroni correction was applied to overcome multiplicity problem. Statistical analyses were performed using the computer program SPSS, version 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Characteristics of the patients and treatment compliance and toxicity profile
Common presenting symptoms were vaginal bleeding in 45 patients (60.8%), followed by vaginal discharge in 16 patients (21.6%), and low backache in 13 patients (17.6%). The median duration of symptoms was 9 months (3–15). The median age of cohort was 52.3 years (32–78). FIGO stage IIB was the predominant stage in 49 patients (66.2%) followed by IIIA in 12 patients (16.2%). Twenty-nine patients (39.1%) had negative pelvic nodal at the time of CCRT. The common site of gross pelvic lymph nodes were as follows: iliac in 21 patients (28.4%), common iliac in 16 patients (21.6%), and gross para-aortic in 8 patients (10.8%). Thirty-six patients (48.7%) were treated with pelvic irradiation, and pelvic and para-aortic irradiation was used in 38 patients (51.3%) (Table 1).
Table 1.
Patient characteristics.
| Variables | CCRT (n=74) |
| Age | 52.3 y (32–78) |
| Comorbidities | |
| Hypertension | |
| Yes | 33 (44.6%) |
| No | 41 (55.4%) |
| Diabetes | |
| Yes | 30 (40.6%) |
| No | 44 (59.4%) |
| Histopathology | |
| Squamous cell carcinoma | 67 (90.5%) |
| Adenocarcinoma | 5 (6.7%) |
| Adenosquamous cell carcinoma | 2 (2.7%) |
| FIGO staging | |
| IIB | 49 (66.2%) |
| IIIA | 12 (16.2%) |
| IIIB | 7 (9.5%) |
| IVA | |
| Radiological primary tumor size | |
| <5 cm | 27 (36.5%) |
| >5 cm | 47 (63.5%) |
| MRI-based nodal involvement | |
| Negative | 29 (39.1%) |
| Iliac | 21 (28.4%) |
| Common Iliac | 16 (21.6%) |
| Para-aortic | 8 (10.8%) |
| Pretreatment hemoglobin | |
| >10 gm/dL | 69 (93.3%) |
| <10 gm/dL | 5 (6.7%) |
| Treatment | |
| EBRT: | |
| Whole pelvis | 45 Gy (42–50.4) |
| Para-aortic | 45 Gy (45–50.4) |
| Parametrial boost | 9 Gy (0–9) |
| HDR-BT: | |
| Dose/Fraction | 7 Gy/fraction |
| Total dose/fraction | 21 Gy/3 |
| Point A BED | 86.4 Gy (80.5–102.7) |
| ICRU 38 rectal point BED | 85 Gy (80.5–100) |
| ICRU 38 bladder point BED | 86 Gy (80.5–102) |
| Concurrent weekly cisplatin cycle: | |
| Dose/wk | 30 mg/m2 |
| Mean cycles | 5 (4–7) |
FIGO: International Federation of Gynecologists and Obstetrics, CCRT: concurrent chemoradiation, MRI: magnetic resonance imaging, EBRT: external beam radiation therapy, BED: biologic effective dose, ICRU: international commission of radiation units, HDR-BT: high dose rate brachytherapy.
The median follow-up time was 5 years (range, 3–5.5 years). Among all 74 patients who received CCRT, the treatment protocol completion rate was 90 % (95% confidence interval [CI], 85–100). The weekly concurrent cycles of cisplatin in both treatment groups were completed in all 74 (100%) patients with no interruption. The median duration of radiation therapy in both groups was 55.5 days (48–58).
The overall grade 3 or 4 acute hematological and nonhematological toxicities were seen in 8 patients (10.8%). Grade 3 or 4 acute gastrointestinal toxicity was seen in 4 patients (5.4%) (Table 2).
Table 2.
Acute and late toxicity profile.
| Toxicity | CCRT (n=74) | |
|---|---|---|
|
| ||
| Acute: | G3 | G4 |
| Hematologic | ||
| Neutropenia | 4 (5.4%) | 0 |
| Thrombocytopenia | 0 | 0 |
| Anemia | 0 | 0 |
| Nonhematologic | ||
| Nausea/Vomiting | 4 (5.4%) | 0 |
| Diarrhea | 0 | 0 |
| Cystitis | 0 | 0 |
| Deranged renal functions | 0 | 0 |
| Deranged liver functions | 0 | 0 |
| Late: | 0 | |
| Chronic cystitis | ||
| Intestinal obstruction | 1 (1.35%) | 0 |
| Proctitis | ||
| Neuropathy/Plexopathy | 0 | |
| Hearing loss | ||
| Renal | 2 (2.7%) | |
CCRT: Concurrent chemoradiation, G: grade.
During the follow-up time of 5 years in the EFCCRT group, 1 patient (1.4%) experienced sub-acute intestinal obstruction. No patient in either group underwent surgery for the radiation-induced damage or died because of the treatment-related side effects.
Response rates, locoregional and distant control, and survival rates
At the time of the last follow-up, 13 patients (17.6%) had locoregional recurrences (Figure 3A). Among those, 6 patients (46.1%) had pelvic recurrences, 5 patients (38.5%) had para-aortic recurrences, and 2 patients (15.4%) had isolated vaginal recurrences that were treated with surgery. Eight patients (72.7%) with pelvic/para-aortic nodal recurrences were treated with salvage chemotherapy and 2 patients (18.1%) received additional para-aortic irradiation. Salvage chemotherapy was generally poorly tolerated.
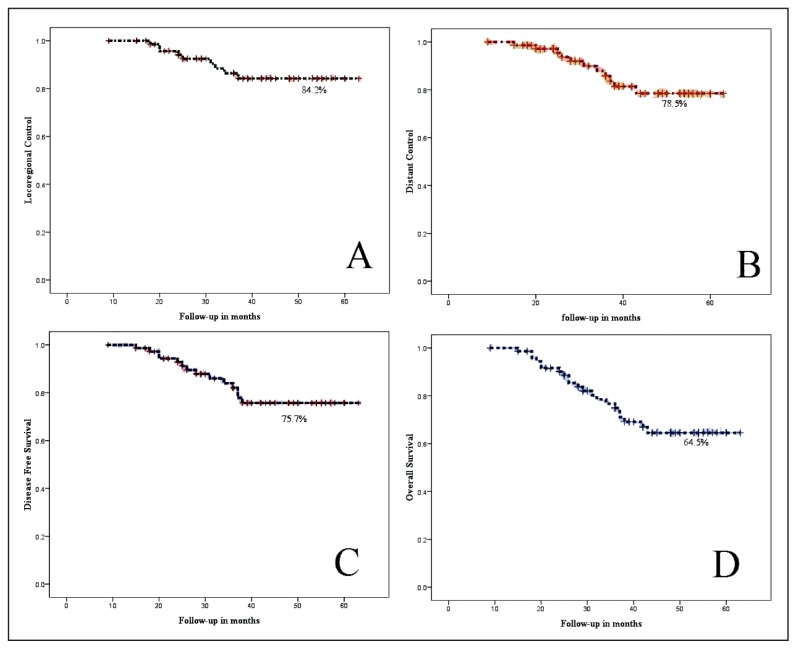
Figure 3.
Five-year (A) locoregional control, (B) distant control, (C) disease-free survival, and (D) overall survival rates in concurrent chemoradiation.
Distant failures were seen in 9 patients (12.1%). The common sites of distant failure were as follows: lungs in 4 patients (44.6%), mediastinal in 3 patients (33.3%), supraclavicular nodes in 1 patient (11.1%), and bone in 1 patient (11.1%) (Figure 3B). The median time to initial locoregional and distant failure was 20 months (range 19–24 months). Combined distant and locoregional failures were observed in 4 patients (18.2%). All distant failures were treated with salvage chemotherapy and radiotherapy for bone and supraclavicular nodes. At the time of analysis, 51 patients were found without disease with overall DFS of 75.7%. Forty-seven patients were alive at the time of analysis with OS of 64.5% (Figure 3C and D).
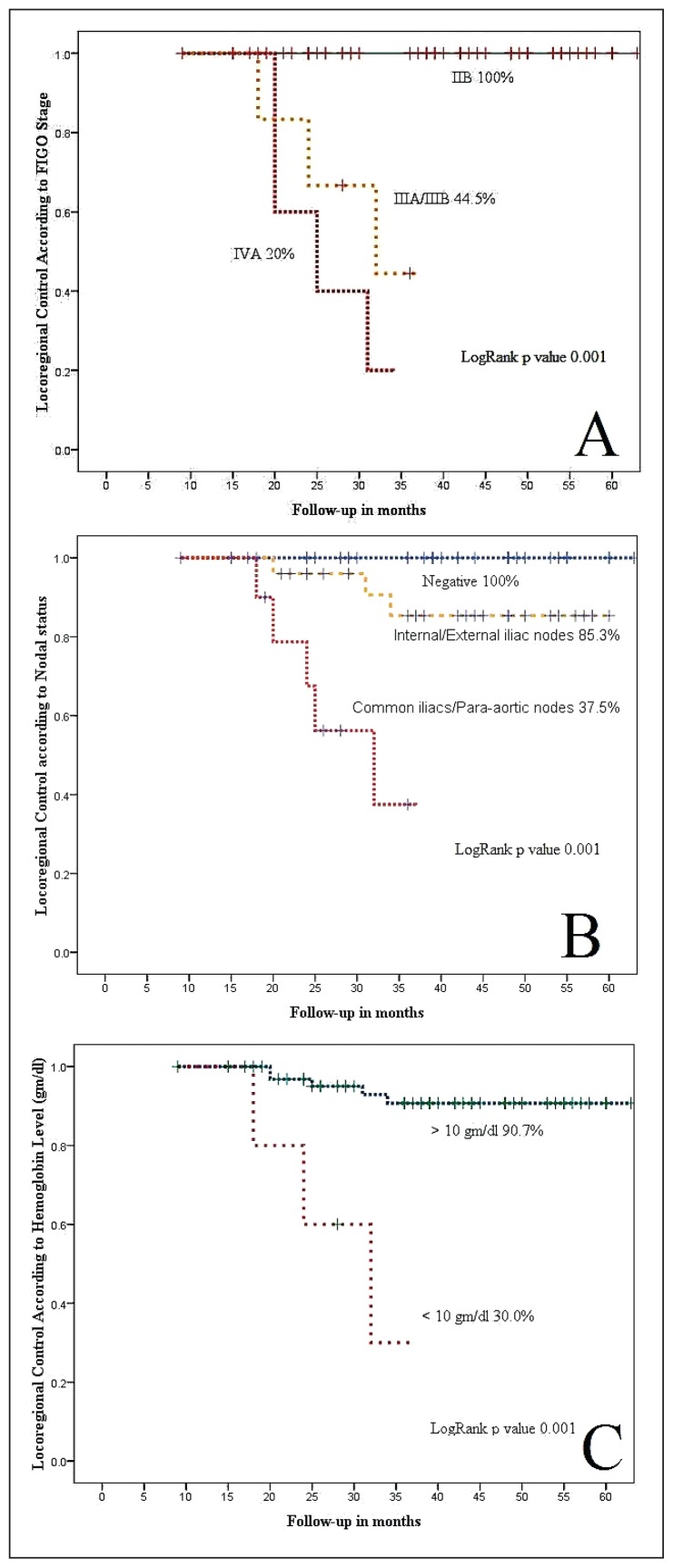
Cox regression model analysis showed that nodal status, FIGO stage, and CCRT duration were important prognostic factors for locoregional and distant control (Figure 4 and Table 3).
Figure 4.
Five-year locoregional control according to (A) FIGO stage, (B) nodal status, and (C) pretreatment hemoglobin levels.
Table 3.
Cox regression model analysis of variables on locoregional, distant control, and disease-free survival rates.
| Overall Survival P value HR (95% CI) |
Disease-Free Survival P value HR (95% CI) |
Distant Metastasis Control P value HR (95% CI) |
Locoregional Control P value HR (95% CI) |
Variable |
|---|---|---|---|---|
|
| ||||
| 0.80 0.80 (0.77–0.98) | 0.70 0.50 (0.10–2.41) | 0.60 1.10 (0.89–2.00) | 0.80 1.80 (0.79–2.10) | Age (<50 y vs > 50 y) |
| 0.04 2.20 (1.60–4.11) | 1.0 1.80 (0.79–2.10) | 0.90 1.80 (0.79–2.10) | 0.90 0.88 (0.67–0.97) | Comorbids (Yes vs No) |
| 0.01 2.56 (1.70–6.50) | 0.01 3.95 (1.91–10.35) | 0.02 4.65 (1.81–9.65) | 0.001 7.21 (3.22–16.30) | FIGO stage (<IIB vs >IIB) |
| 0.03 3.0 (2.40–7.25) | 0.01 4.01 (2.21–11.59) | 0.03 3.66 (1.75–9.36) | 0.001 6.34 (4.52–11.34) | N stage (N0 vs N1) |
| 0.50 1.10 (0.89–2.00) | 0.40 0.78 (0.23–2.38) | 0.50 1.10 (0.89–2.00) | 0.05 1.10 (0.96–1.20) | Hemoglobin level (<10 gm/dL vs >10 gm/dL) |
| 0.40 1.21 (1.10–2.10) | 0.70 1.21 (1.10–2.10) | 0.60 1.10 (0.89–2.00) | 0.40 1.21 (1.10–2.10) | Cell type (squamous vs nonsquamous) |
| 0.50 1.10 (0.98–1.20) | 0.60 1.10 (0.89–2.00) | 0.90 0.88 (0.67–0.97) | 0.03 3.21 (2.45–7.85) | Treatment duration (<54 vs >54 d) |
FIGO: International Federation of Gynecologists and Obstetrics, OR: odds ratio, CI: confidence intervals, N: nodal status.
DISCUSSION
Pelvic irradiation with concurrent chemotherapy is considered the standard treatment by many North American and European teams for the treatment of locally advanced cervical cancer, which has resulted in survival gain of 10% to 20%.7 The 10-year results of a Radiation Therapy Oncology Group (RTOG-97-20) trial, which compared pelvic irradiation with para-aortic and pelvic irradiation, have shown an improvement in the survival gain of 11% in the prophylactic paraaortic and pelvic irradiation group, but no difference in locoregional control.8,9 A subsequent another RTOG trial (RTOG 90-01) compared prophylactic extended-field radiotherapy versus pelvic irradiation with chemotherapy in locally advanced cervical cancer patients. This showed 5-year OS rates for patients treated with pelvic irradiation and concurrent chemotherapy, which was significantly greater than for patients treated with the extended-field radiation alone (67% vs 41% at 8 years), and pelvic irradiation with concurrent chemotherapy resulted in a 51% reduction in the risk of recurrence and a 52% reduction in the risk of death.10
To the best of our knowledge, our single institutional study is the first one to report the 5-year treatment outcomes of 3DCRT and HDR brachytherapy–based concurrent chemoradiation in FIGO IIB–IVA locally advanced cervical cancer in Saudi Arabia. The 5-year locoregional control, distant control, and DFS and OS rates in our study were 84.3%, 78.5%, 75.7%, and 64.5%, respectively, which are similar or better in some outcomes than the previously reported trials. 11,12 The course of treatment was well tolerated in our patients, and hematological and nonhematological toxicities were minimal and better than those reported in other trials of using CCRT. The possible explanation for lower toxicity profile and better outcomes in our study is as follows: (a) majority of our patients had FIGO Stage IIB, pelvic nodes negative, (b) surgical staging for para-aortic lymph nodes was not carried out as reported in other trials that enhances the gastrointestinal toxicity, instead, (c) optional FDG/PET was performed, (d) moderate doses of weekly cisplatin, and (e) use of better radiation techniques (3DCRT and HDR brachytherapy). We used radiologic (CT/MRI) staging along with FIGO staging, as the tumor size and lymph node involvement are also important prognostic factors that influence the locoregional control and survival as seen in our study and mentioned by other related studies.13,14 The reason for the relatively lower OS rate is that the majority of our cohort was hypertensive (44.6%) and diabetic (59.4%), which have been known as important prognostic factors for OS in cervical cancer.15
Further, our results have shown that in addition to the FIGO stage, nodal status and treatment duration are also significant prognostic factors for locoregional control; however prognostic factors for distant control and DFS in our study were FIGO stage and nodal status. Pretreatment hemoglobin <10 gm/dL has been considered an important prognostic factor for OS,16 but we could not see its impact on locoregional control and OS in our patients. The total duration of concomitant chemoradiation has been suggested to be restricted within 56 days to increase locoregional control and OS;17 however, the majority (90%) of our patients finished treatment within 55 days, which reflects the better locoregional control and DFS in our patients.
The strengths of our study were as follows: reasonable sample size of Saudi women with locally advanced cervical cancer, use of modern radiation therapy techniques (3DCRT and HDR brachytherapy), and longer follow-up period. The limitations of our study were as follows: retrospective data and the majority of cohort was with comorbidities and gross pelvic lymphadenopathy, which warrants the need of diabetic and hypertension awareness campaigns along with routine cytological screening in Saudi Arabia.
In conclusion, the combined chemotherapy and radiation therapy in Saudi women with locally advanced cervical cancer results into better locoregional control, distant control, and DFS, which is consistent with international data. However, in future, trials incorporating IMRT in locally advanced cervical shall be done to see additional benefit of combined chemoradiation.
REFERENCES
- 1.Makoha FW, Raheem MA. Gynecological cancer incidence in a hospital population in Saudi Arabia: the effect of foreign immigration over two decades. J Obstet Gynaecol Res. 2008;34:538–42. doi: 10.1111/j.1447-0756.2008.00735.x. [DOI] [PubMed] [Google Scholar]
- 2.Jamal A, Al-Maghrabi JA. Profile of Pap smear cytology in the Western region of Saudi Arabia. Saudi Med J. 2003;24:1225–9. [PubMed] [Google Scholar]
- 3.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, 3rd, et al. Cisplatin, radiation and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical cancer. N Eng J Med. 1999;340:1154–61. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 4.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin based radiotherapy and chemotherapy for locally advanced cervical cancer. N Eng J Med. 1999;340:1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 5.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stages IIB–IV carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group Study. J Clin Oncol. 1999;17:1339–48. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 6.El-Senoussi M, Bakri Y, Amer MH, DeVol EB. Carcinoma of the uterine cervix in Saudi Arabia: experience in the management of 164 patients with stage-I & -II disease. Int J Radiat Oncol Biol Phys. 1998;42:91–100. doi: 10.1016/s0360-3016(98)00166-7. [DOI] [PubMed] [Google Scholar]
- 7.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Eng J Med. 1999;340:1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 8.Rotman M, Choi K, Guse C, Marcial V, Hornback N, John M. Prophylactic irradiation of the para-aortic lymph node chain in stage IIB and bulky stage IB carcinoma of the cervix, initial treatment results of RTOG 7920. Int J Radiat Oncol Biol Phys. 1990;19:513–21. doi: 10.1016/0360-3016(90)90475-y. [DOI] [PubMed] [Google Scholar]
- 9.Rotman M, Pajak TF, Choi K, Clery M, Marcial V, Grigsby PW, et al. Prophylactic extended-field irradiation of para-aortic lymph nodes in stages IIB and bulky IB and IIA cervical carcinomas. Ten-year treatment results of RTOG 79–20. JAMA. 1995;274:387–93. [PubMed] [Google Scholar]
- 10.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and paraaortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–80. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 11.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–13. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 12.Small W, Jr, Winter K, Levenback C, Iyer R, Gaffney D, Asbell S, et al. Extended-field irradiation and intracavitary brachytherapy combined with cisplatin chemotherapy for cervical cancer with positive para-aortic or high common iliac lymph nodes: results of Arm 1 of RTOG 0116. Int J Radiat Oncol Biol Phys. 2007;68:1081–7. doi: 10.1016/j.ijrobp.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Kodaira T, Fuwa N, Toita T, Nomoto Y, Kuzuya K, Tachibana H, et al. Comparison of prognostic value of MRI and FIGO stage among patients with cervical carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:769–77. doi: 10.1016/s0360-3016(03)00007-5. [DOI] [PubMed] [Google Scholar]
- 14.Scheidler J, Hricak H, Yu KK, Subak L, Segal MR. Radiological evaluation of lymph node metastases in patients with cervical cancer. A metaanalysis. JAMA. 1997;278:1096–101. [PubMed] [Google Scholar]
- 15.Cetina L, Garcia-Arias A, de Uribe MJ, Candelaria M, Rivera L, Oñate-Ocaña L, et al. Concurrent chemoradiation with carboplatin for elderly, diabetic and hypertensive patients with locally advanced cervical cancer. Eur J Gynaecol Oncol. 2008;29:608–12. [PubMed] [Google Scholar]
- 16.Demirci S, Ozsaran Z, Ozsaran A, Yavas F, Demircioglu B, Hanhan M, et al. Evaluation of treatment results and prognostic factors in early-stage cervical carcinoma patients treated with postoperative radiotherapy or radiochemotherapy. Eur J Gynaecol Oncol. 2012;33:62–7. [PubMed] [Google Scholar]
- 17.Petereit DG, Sarkaria JN, Chappell R, Fowler JF, Hartmann TJ, Kinsella TJ, et al. The adverse effect of treatment prolongation in cervical carcinoma. Int J Radiat Oncol Biol Phys. 1995;32:1301–7. doi: 10.1016/0360-3016(94)00635-X. [DOI] [PubMed] [Google Scholar]