Figure 1.
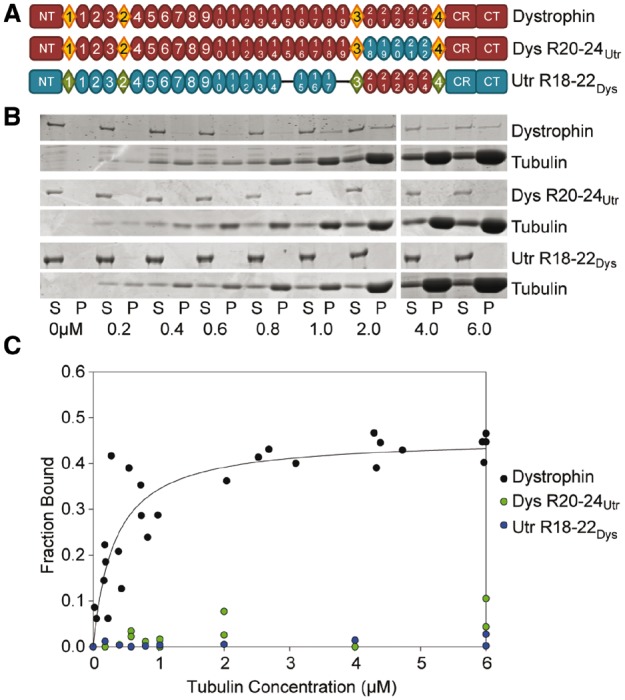
Domain structure and microtubule binding properties of dystrophin/utrophin hybrid constructs. (A) Schematic representation of full-length dystrophin and utrophin hybrid constructs used in this study. Ovals represent spectrin-like repeats, diamonds represent hinge regions. NT, N-terminus; CR, cysteine-rich domain; CT, C-terminus. Blue ovals represent portions of utrophin swapped into dystrophin. Red ovals represent dystrophin repeats swapped into utrophin. (B) Representative coomassie blue stained SDS-PAGE microtubule cosedimentation gels for dystrophin, Dys 20–24Utr, and Utr 18–22Dys. S, supernatant (fraction not bound to microtubules); P, pellet (fraction bound to microtubules). Tubulin dimer concentration is listed below each pair of lanes. (C) Microtubule binding curves. Substituting dystrophin repeats 20–24 for utrophin repeats 18–22 ablates-specific microtubule binding. However, substituting utrophin repeats 18–22 for dystrophin repeats 20–24 does not confer microtubule binding to utrophin. Full-length dystrophin data was previously published and shown here for reference (9).
