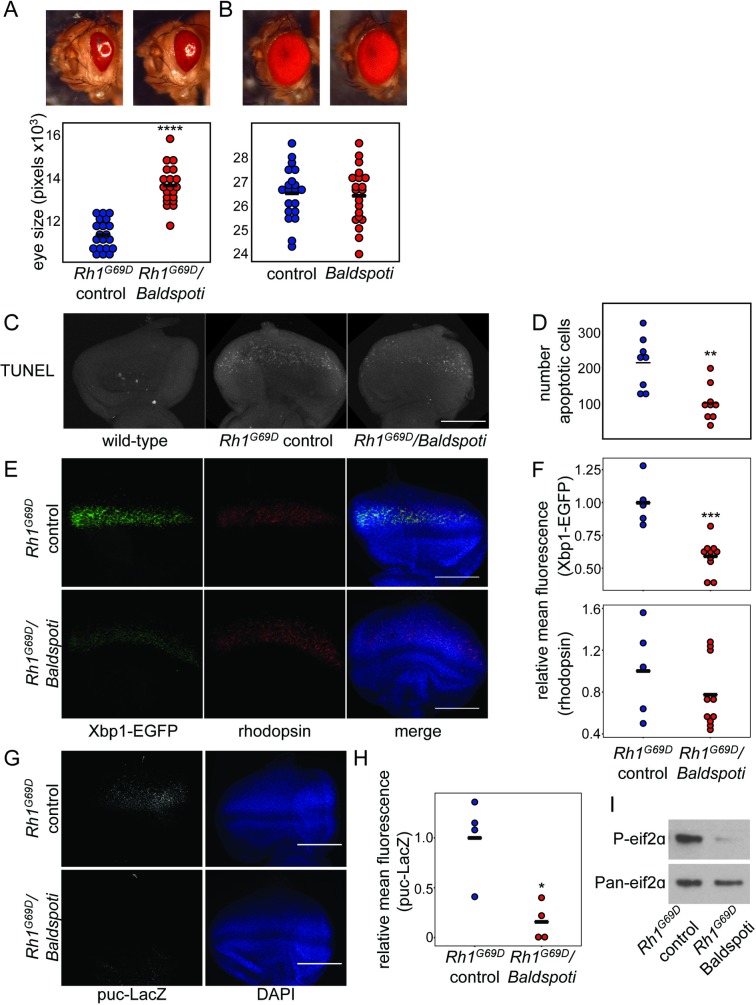
Fig 2. Loss of Baldspot in the Rh1G69D eye disc alleviates ER stress and apoptosis.
(A) Degeneration caused by overexpression of Rh1G69D is partially rescued by RNAi knockdown of Baldspot (Rh1G69D/Baldspoti vs Rh1G69D controls). This rescue was significant when eye size was quantified, as indicated by a larger eye in Rh1G69D/Baldspoti flies (14043 ± 960 pixels in Rh1G69D/Baldspoti flies and 11942 ± 473 pixels in controls). (B) Loss of Baldspot on a wild-type background had no effect on eye phenotype (26478 ± 1191 pixels and 26582 ± 1110 pixels in controls). (C) Rh1G69D/Baldspoti eye discs display reduced apoptosis levels compared to Rh1G69D controls, as measured by TUNEL staining. (D) Cell death was quantified by counting the number of TUNEL positive cells in each eye disc. (E) Rh1G69D/Baldspoti eye discs had reduced expression of Xbp1-EGFP as compared to Rh1G69D controls, while rhodopsin levels were unchanged. Eye discs were dissected from wandering L3 larvae expressing Rh1G69D and UAS-Xbp1-EGFP, stained for rhodopsin and GFP as an indicator of ER stress, and counterstained with 4',6-diamidino-2-phenylindole (DAPI). (F) Xbp1-EGFP levels were significantly reduced in Rh1G69D/Baldspoti eye discs (0.59 ± 0.13 fold-change from controls) as compared to Rh1G69D controls (1.00 ± 0.18). Rh1 levels were not changed (0.78 ± 0.33 fold-change from 1.00 ± 0.44 in controls). (G) Rh1G69D/Baldspoti eye discs had reduced expression of puc-LacZ as compared to Rh1G69D controls. (H) When quantified, LacZ levels were significantly reduced in Rh1G69D/Baldspoti eye discs (0.16± 0.19 fold-change from controls) as compared to Rh1G69D controls (1.00 ± 0.41). (I) Rh1G69D/Baldspoti eye discs had reduced expression of P-eif2α as compared to Rh1G69D controls. Fluorescence was quantified using mean fluorescence from the image stack as calculated in Image J. Scale bars = 0.1 mm. * P < 0.05, ** P < 0.005, *** P < 0.0005, **** P < 0.0001.