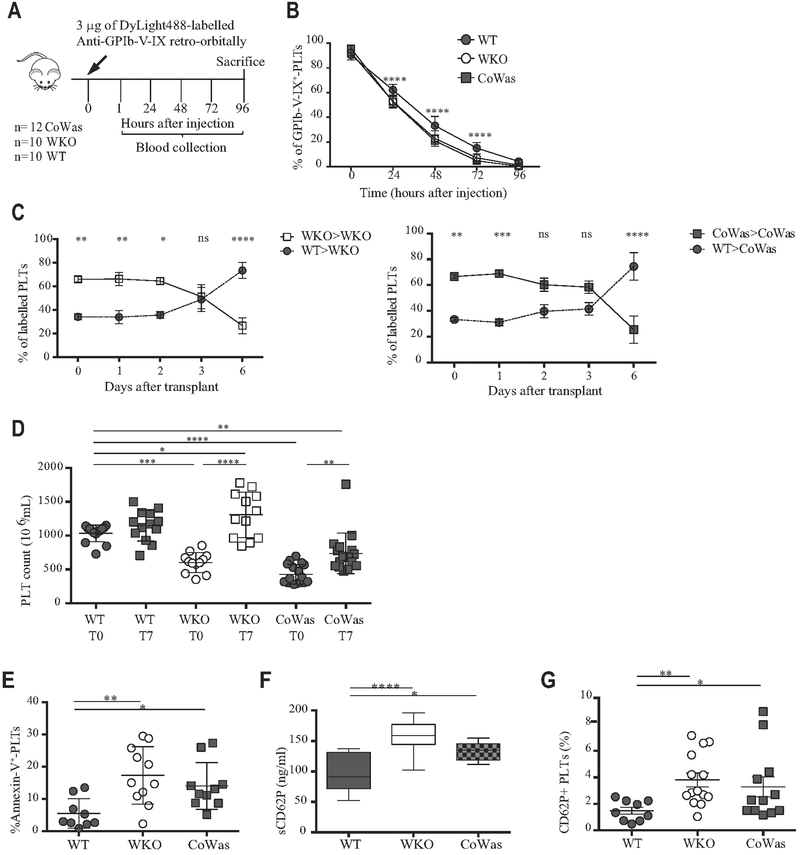
FIG 3.
Peripheral elimination of platelets. A, Experimental plan of in vivo platelet half-life detection. B, Platelet half-life was analyzed as the percentage of GPIb-V-IX+ platelets 15 minutes after injection (time 0) and at different time points after injection. C, Differentially labeled WT and Was−/− platelets have been mixed in a 30%/70% ratio and injected into recipients. Was−/− platelets isolated from WKO mice have been transferred to WKO mice (left panels), whereas Was−/− platelets isolated form CoWas mice have beentransferred into CoWas mice(rightpanels; n = 5). D, Platelet counts havebeen evaluatedbefore clodronate liposome injection (T0) and after7 days (T7). E, Apoptotic platelets are expressed as Annexin V+ platelets. F, sCD62P (in nanograms per milliliter) plasma levels (n = 10). G, The graph shows CD62P+ platelets evaluated by means of flow cytometry and expressed as a percentage of the total platelet population. In all graphs each dot represents a different mouse from 1 (Fig 3, C), 2 (Fig 3, E and F), 3 (Fig 3, B and G), or 6 (Fig 3, D) independent experiments. All graphs report means ± SDs. Fig 3, C, reports means ± SEMs. Statistical analysis was performed with 1-way ANOVA(Fig 3, D-F) or 2-way ANOVA (Fig 3, B and C) and the Bonferroni postcorrection test. Fig 3, G, reports means ± SDs and has been analyzed with the Mann-Whitney test. *P < .05, **P < .005, ***P < .001, and ****P < .0001. ns, Not significant; PLT, platelets.