Figure 2.
Increases in Short-Term Facilitation with Distance along a Dendrite Boost Distal Synaptic Integration
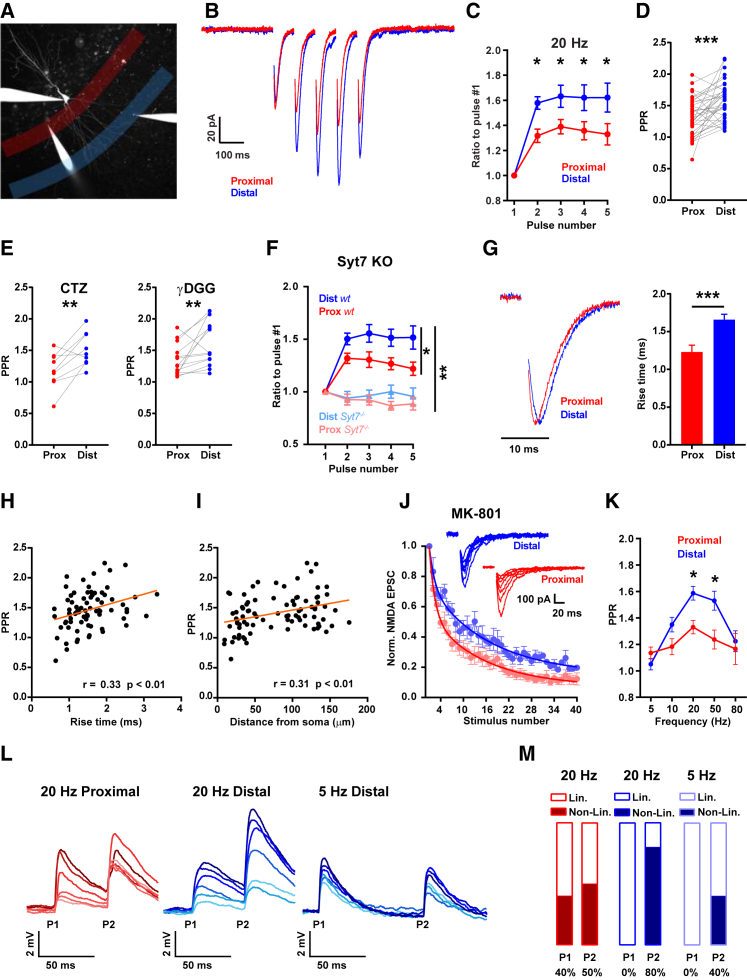
(A) Whole-cell patch-clamp technique was used to record synaptic currents and fill CA1 pyramidal cells with a fluorescent dye to image its structure. Two stimulating pipettes were placed in the proximal (red) and distal (blue) extracellular domains of the basal dendritic tree of pyramidal cells to stimulate the local fibers.
(B) Single cell example of average EPSC responses (average of 20 individual sweeps) to trains of 5 pulses at 20 Hz delivered to the proximal region (red trace) and the distal region (blue trace). The distal response shows greater facilitation compared to the proximal one.
(C) Normalized average peak EPSC amplitudes for distal and proximal responses show greater sustained facilitation during a 5 pulse train (20 Hz) for distal synapses, n = 35 cells, multiple t tests with p values adjusted with the Holm-Sidak method, p < 0.05.
(D) Paired-pulse ratios (PPRs) for each individual cell recorded at distal and proximal synapses. The majority (29/35) of cells display greater facilitation in the distal domain, n = 35 cells, p = 0.0003 two-tailed paired t test.
(E) Distal increase in PPR is not ascribable to postsynaptic AMPA receptor desensitization (prevented by CTZ application) or to AMPA receptor saturation (avoided with γDGG application). Distal PPR is greater than proximal PPR with CTZ (n = 9 cells, p = 0.01), and γDGG (n = 13 cells, p < 0.01), two-tailed paired t test. Two-way ANOVA to test PPRs in control (D), CTZ, and γDGG conditions together shows no significant interaction, p = 0.84, indicating that the drugs have no effect on STP properties.
(F) Full Synaptotagmin7 KO eliminates facilitation and proximo-distal STP differences. Triangles in lighter colors are from Syt7KO mice, n = 9 cells, circles in darker colors are littermate wild-type mice, n = 12 cells. For WT mice, proximal facilitation is lower than distal, multiple t tests, p < 0.05. WT facilitation is greater than Syt7KO facilitation, p < 0.01 multiple t tests.
(G–I) Distal synaptic EPSCs take longer to reach the soma. (G) Left panel: normalized trace for a proximal and distal EPSC response showing the delayed kinetics of the distal compared to the proximal synaptic current. Right panel: the rise time constant of the EPSCs was significantly higher in distally triggered events, n = 49 cells, p < 0.001 Wilcoxon signed rank test. (H) Longer rise times correlate with the amount of facilitation, Spearman’s correlation. (I) PPR is higher when the stimulation electrode is placed further away from the soma, measured as distance along the dendrite, Spearman’s correlation.
(J) Proximal synapses display greater Pr than distal synapses. After MK-801 bath application, the normalized amplitude of EPSCs from proximally stimulated synapses decay faster, following successive stimulations, than distal ones; n = 8 cells. Data points were fit with a double exponential function (filled lines). Insets are example traces of 7 successive NMDA mediated EPSCs.
(K) Frequency tuning curve showing PPRs for all frequencies tested. Distal PPRs (second stimulus only) increase significantly in the 20 Hz (n = 35) same as (D), and 50 Hz range (n = 20), p < 0.05, multiple t tests with Holm-Sidak adjusted p values. 5 Hz (n = 13 cells), 10 Hz (n = 16), 80 Hz (n = 9).
(L–M) Short-term facilitation contributes to dendritic non-linear events in distal domains. (L) Current-clamp example traces (red proximal, blue distal stimulation) in response to a paired pulse, of increasing stimulus intensity (lighter color shades represent lower intensity). (M) The proportion of supra-linear events (at least 2 mV above the expected response) for distal synapses is greatly increased for the second pulse (P2) following facilitation at 20 Hz (0 events in P1, 8 events in P2, n = 10), while supra-linear events were detected to the first pulse (P1) for proximal stimulations (n = 10, 4 in P1, 5 in P2). At 5 Hz, where STP is absent, distal synapses had fewer supra-linear events (n = 5, 0 in P1, 2 in P2). See also Figures S2 and S3. Data are represented as mean ± SEM.