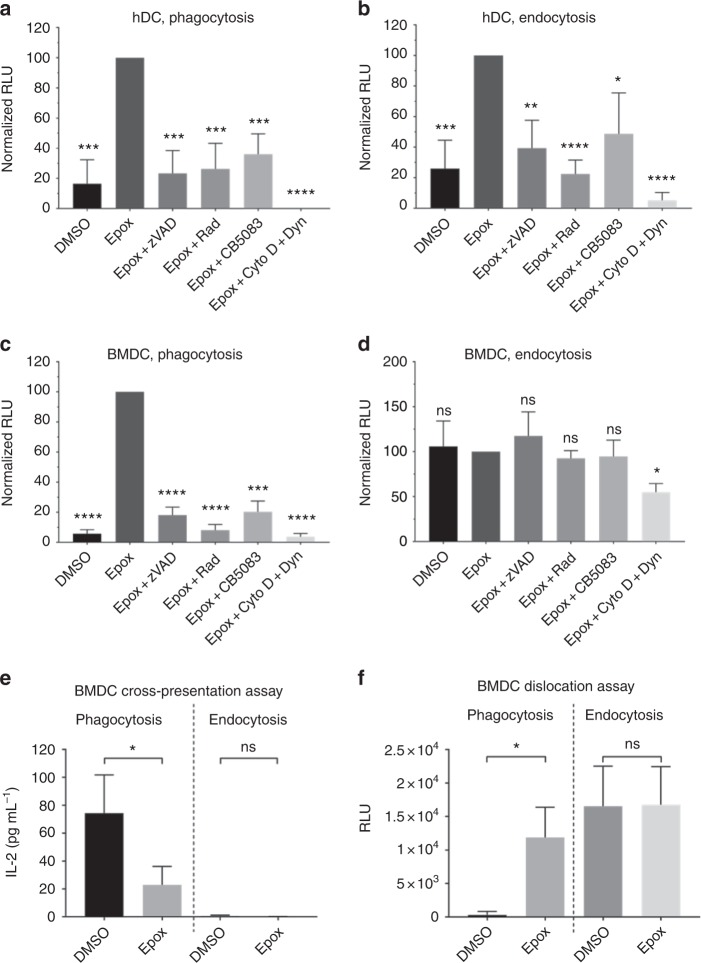
Fig. 4.
ddRLuc-Fc dislocation and cross-presentation by primary dendritic cells. a–d Primary dendritic cells were incubated with ddRLuc-Fc-bound 3 μm latex beads for phagocytosis (a, c) or soluble ddRLuc-Fc at 100 μg mL−1 for endocytosis (b, d). Cell lysates were prepared, processed, and analyzed as described in Fig. 2. Human DCs (hDCs) (a, b) were harvested 6 h after phagocytosis/endocytosis, while mouse bone marrow-derived DCs (BMDCs) (c, d) were harvested after 3 h. Drugs were used at the following concentrations: 800 nM Epox, 20 μm zVAD, 32 μm Rad, 1 μm CB5083, 2.5 μg mL−1 Cyto D plus 100 μm Dyn. e, f Mouse BMDCs were incubated with ddRLuc-FcOVA-bound 3 μm latex beads at a 30:1 bead:cell ratio for phagocytosis (left panels) or with 50 μg mL−1 soluble ddRLuc-Fc for endocytosis (right panels) in the presence or absence of 800 nM Epox. Cells were harvested at 3 h, and assessed for luciferase activity (e) or stimulation of IL-2 secretion after an additional overnight incubation with the B3Z hybridoma (f). Bars represent the mean +/−s.d. of at least three independent experiments per treatment (paired two-tailed t-test, *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, not significant)