Abstract
Pyroptosis, characterized by proinflammation, has been defined as a new type of programmed cell death in recent years. Inflammasomes are activated by the corresponding pathogen-associated molecular patterns (PAMPS) or damage-associated molecular patterns (DAMPS), followed up by the cleavage of pro-interleukin-1β (pro-IL-1β), pro-interleukin-18 (pro-IL-18) and gasdermin D. The N-terminal fragment of gasdermin D gives rise to the destruction of cell membrane, leading to cell rupture as well as the efflux of proinflammatory cytokines. Recent studies have shown that pyroptosis is associated with a variety of diseases due to its proinflammation effect and the dysfunction of related cells. The relationship between pyroptosis and associated diseases is described in this review.
Keywords: Pyroptosis, disease, inflammation, cell death
Introduction
Pyroptosis, a form of programmed cell death, is distinguished from apoptosis and necroptosis due to its feature of proinflammation and its dependence on specific pyroptopic caspases to induce cell death [1,2]. Pyroptosis is mediated by pyroptotic caspases including caspase-1 (both in humans and mice), human caspase-4, human caspase 5, and mouse caspase-11 [3]. Caspase-4, caspase 5 and caspase-11 are activated by their direct binding to lipopolysaccharides (LPS), while caspase-1 is mediated by pyroptopic inflammasome sensors [4,5]. The upregulated activation of pyroptopic caspases triggers cell swelling and further rupture through the cleavage of gasdermin D [6]. The cell contents, along with proinflammatory cytokines flow out as a result of the dysfunction of cell membrane, causing local or systemic inflammatory responses.
Given its two major effects, cell dysfunction and proinflammation, pyroptosis is thought to participate in several diseases. As one of the most common pathological processes, inflammation is involved in generation and development of multiple types of diseases, such as infectious disease, immune disorders, and cancer [7]. Infectious disease and immune disorders produce PAMPs and DAMPs, which can be recognized by inflammasome sensors and can further trigger inflammation. As for cancer, inflammation exists both at the generation and progression stage, stimulating its deterioration and metastasis [7]. Also, cell dysfunction plays an important role in neurodegenerative disease [8], cardiovascular disease [9], and AIDS [10]. The mortality of neurons partially accounts for the degeneration of the nerve system [8], while cardiovascular disease are often associated with the progressive endothelial dysfunction derived from the death of endotheliocytes [9]. Although the association between AIDS and inflammation is apparent, the dysfunction of CD4+ T-lymphocytes is much more important in its pathology [10].
Molecular mechanism of pyroptosis
Caspase-1 dependent pyroptosis
The initiation of caspase-1 dependent pyroptosis is the recognition between inflammatory sensors and molecular patterns. The rat NLRP1 and the mouse NLRP1b were identified as substrates of anthrax lethal factors through the cleavage of certain sites [11,12]. Furthermore, a negative correlation was found between the concentration of cytosolic ATP and the activation of NLRP1b [13], indicating that NLRP1b is under the regulation of both PAMPs and DAMPs. NLRP3 can sense plentiful patterns including bacterial RNA, toxins, ATP, etc [14,15]. The activation of NLRP3 is partially related to mitochondrial damage which has a key role in reactive oxygen species [16]. Also, NEK7 was identified as an essential protein to mediate the assembly and function of NLRP3-related inflammasome in response to potassium efflux [17]. NAIPs contribute to the connection between PAMPs and NLRC4 [18,19]. Once NAIP2 recognizes and binds to PrgJ, it becomes activated and is then allowed to recruit NLRC4 [19]. However, flagellin is found related to NAIP5-NLRC4 [19]. Absent in melanoma 2 (AIM2) can recognize the cytosolic dsDNA through the HIN200 domain [20], while pyrin functions to sense the inactivation of Rho GTPases mediated by Rho-inactivating toxins [21,22], both resulting in the upregulated activation of caspase-1.
Once inflammatory sensors are activated, certain sensors are prepared for the formation of inflammasome [23]. Among the activated pyroptopic inflammatory sensors, NLRP3, AIM2 and pyrin recruit pro-caspase-1 with the help of ASC [24], an adaptor protein which consists of a pyrin domain (PYD) and a caspase recruitment domain (CARD). Nevertheless, NLRP1b and NLRC4 might directly recruit pro-caspase-1 since both of them possess a CARD domain [25]. The activation of caspase-1 is obtained from the assembly of inflammasome, followed up by the cleavage of gasdermin D [6]. Gasdermin D is cleaved into two fragments, and the N-terminal fragment squeezes into cell membrane to form open pores [26-28], destroying physiological activity of membrane as well as causing cell swelling and rupture. The activated caspase-1 can also cleave pro-IL-1β and pro-IL-18 into IL-1β and IL-18 [29,30], stimulating local or systematic inflammation (Figure 1).
Figure 1.
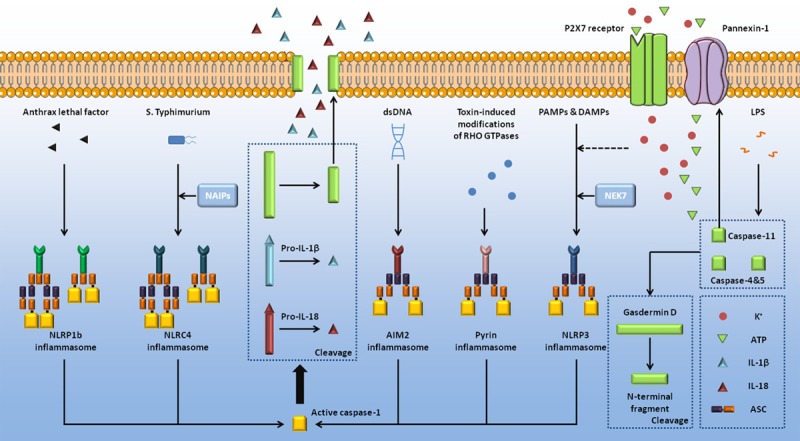
Molecular mechanism of pyroptosis. Induced by certain PAMPs and DAMPs, pyroptopic sensors form a macromolecular complex together with caspase-1, as well as ASC sometimes [23,24]. NAIP proteins [18,19] and NEK7 [17] are required during the formation of NLRC4 inflammasome and NLRP3 inflammasome. The activated caspase-1 can directly cleave gasdermin D [6], of which the N-terminal fragment forms pores on the host cell membrane, mediating the release of cell contents [26-28]. Pro-IL-1β and pro-IL-18 are also cleaved by caspase-1, initiating local or systematic inflammation [29,30]. Human caspase-4, human caspase-5, and mouse caspase-11 can bind to LPS [31-33], and then cause the cleavage of gasdermin D [6], consistent with caspase-1. Caspase-11 can also cleave pannexin-1, inducing the release of cytoplasmic ATP [35]. Extracellular ATP functions as ligand of P2X7 receptor, followed by potassium efflux which initiates the activation of NLRP3 [16].
Caspase-1 independent pyroptosis
In addition to caspase-1, caspase-4, caspase-5, and caspase-11 are also involved in the initiation of pyroptosis. These three caspases can directly bind to LPS, stimulating the activation of caspases [31-33]. Consistent with the caspase-1 dependent pyroptosis, gasdermin D is cleaved at the same site [34]. Besides, a new way of caspase-11 dependent pyroptosis was discovered recently that pannexin-1 is cleaved by activated caspase-11, inducing the release of cytosolic ATP [35,36]. P2X7 receptor, a ATP-gated channel, opens in response to the concentration of extracellular ATP, inducing potassium efflux [35]. Caspase-11-induced activation of pannexin-1 can also trigger potassium efflux, which in turn stimulates NLRP3-mediated pyroptosis [16].
Pyroptosis and disease
Pyroptosis and infectious disease
In pyroptosis pathways, PAMPs and LPS can be recognized by corresponding inflammasomes and caspases respectively to activate downstream pyroptosis pathways. Thus infection with pathogens is a primary way to trigger pyroptosis. It was found that AIM2 instead of NLRP3 plays an important role in staphylococcus aureus infection along with regulating the release of IL-1β [37]. Pereira et al. [38] discovered that flagellin-deficient legionella pneumophila, compared with wild-type or motor-type deficient ones, showed enhancement in its replication, indicating that flagellin was necessary for the NLRC4-dependent pyroptosis. In Lei et al.’s study [39] on the pathogenesis of enterovirus 71, it was found that enterovirus protease 3C could cleave gasdermin D. The cutting site is different from that induced by caspases, leaving the N-terminal fragment physiologically inactive thus disabling the downstream pyroptosis pathways. Enterovirus 71 escaped the resistance mechanism of cell immune system in hosts by directly destroying key factors in pyroptosis pathways, providing a new perspective to re-evaluate the resistance of pathogens to the pyroptosis of hosts.
Pyroptosis and chronic inflammation as well as autoimmune disease
In chronic inflammation and autoimmune disease, inflammatory factors can exhibit balance disorders. In addition, the initiation of chronic inflammation and autoimmune disease may be due to the PAMPs or DAMPs, both also recognized as the initiation of pyroptosis. Therefore, pyroptosis is closely related to chronic inflammation and autoimmune disease. Palacios-Macapagal et al. [40] studied immune responses to necrotic liver injury in vivo and found that necrotic hepatocytes could induce eosinophil aggregation, IL-1β and IL-18 secretion, degranulation and cell death, and this phenomenon could be blocked by caspase-1 inhibitor. This reaction is represented by eosinophils increase in large areas, suggesting that pyroptosis is the way in which eosinophils responded to massive hepatocytes death. Maltez et al. [41] discovered that NLRC4 was essential for the prevention of Chromobacterium violaceum infection, and the classic pyroptosis pathway it activated was the main mechanism of liver protection. In addition, in the liver tissue, the release of IL-18 cooperated with natural killer cells and showed an effective protection effect. Cryopyrin-associated periodic syndromes (CAPS) is a rare inherited auto-inflammatory syndrome associated with mutations in coding residues adjacent to the Ser295 locus of NLRP3 [42]. In the study of CAPS, it was discovered that by PGE2 signaling, protein kinase A (PKA) directly phosphorylated NLRP3 and inhibited its activation, and this phosphorylation process was closely associated with the Ser295 on NLRP3, suggesting that negative regulation of Ser295 was critical to the inhibition of NLRP3 in the case of CAPS pathology [43].
Pyroptosis and neurodegenerative disease
Neurodegenerative disease are due to the gradual loss of function and even death of neurons, a process in which pyroptosis has been found involved [8]. High expression of caspase-1 was found in the brains of patients with Alzheimer’s disease [44]. NLRP3(-/-) or Caspase-1(-/-) mice carrying mutations associated with familial Alzheimer’s disease acquired partially protection from loss of spatial memory and other symptoms associated with Alzheimer’s disease. Also the activity of caspase-1 and IL-1β in the mice’s brains was found decreased, as well as the increased clearance of amyloid-β. These findings revealed that NLRP3-mediated pyroptosis is critical in the pathogenesis of Alzheimer’s disease and proved the feasibility of intervention cure by inhibiting NLRP3. Zhou et al. [45] discovered in their study of Parkinson’s disease that α-synuclein, a pathological feature, could activate NLRP3 and release IL-1β after its endocytosis by microglia, thus aggravating the disease. It is noteworthy that they found NLRP3 as the targeted gene of microRNA-7 where microRNA-7 suppressed the activity of NLRP3. Thus using microRNA-7 to inhibit NLRP3 and as so inhibiting the pathogenesis of Parkinson’s disease could be considered as a new perspective for treatment.
Pyroptosis and cardiovascular disease
Mass mortality of endothelial cells, which leads to vascular endothelium dysfunction, is a major cause for cardiovascular disease. Previous studies [46] on hyperlipidemia and atherosclerosis have reported that in endothelial cells oxidized lipids could activate caspase-1, and then cause pyroptosis. Compared with the control group, the symptom of early atherosclerosis caused by hyperlipidemia was alleviated in caspase-1-deficient mice. Similarly, the loss of endothelial cell function in patients with hypercholesterolemia is also associated with NLRP3-induced pyroptosis. Zhang et al. [47] reported that cholesterol crystal could increase NLRP3 activity in endothelial cells, thereby enhancing the inflammatory response of endothelial cells and process of pyroptosis, which resulted in coronary artery endothelium dysfunction. Moreover, cardiovascular disease is associated with heavy metal ions as well. Cadmium (Cd) is an important and ubiquitous environmental pollutant, which may lead to the cardiovascular system dysfunction by targeted damage of endothelial cells [48]. Thus, Cd is partially relative with cardiovascular disease, such as atherosclerosis and hypertension. A study by Chen et al. [49] showed that Cd could stimulate the production of reactive oxygen species in mitochondria and cytosol of human umbilical vein endothelial cells. Reactive oxygen species are recognized as DAMPs by NLRP3 and initiate downstream pyroptosis, causing endothelial cells damage and vascular dysfunction. These researches demonstrate that there exists a common process in pyroptosis associated cardiovascular disease - endothelial cells sense danger signals and induce NLRP3-dependent pyroptosis, causing mass mortality as well as destroying the vascular endothelium function. Therefore, NLRP3-inhibitory drugs may show effectivity in this kind of treatment.
Pyroptosis and cancer
Because of the high risk and the difficulty to cure, cancer is acquiring more and more attention all over the world. This part will interpret cancer just from pyroptosis angle. In some types of cancer, there is an inflammatory condition before a deterioration occurs [7]. However, in other types of cancer, carcinogenic changes can induce inflammatory microenvironment that promotes tumor progression [7]. Therefore, inflammatory caused by pyroptosis may be related to the occurrence and development of cancer. On the other hand, ‘eternal life’ of cancer cells is one of the major features of cancer, which may due to the escape from cell life cycle [50] as well as the inhibition of pyroptosis. Recent evidence [51,52] suggests that caspase-1 activity was inhibited in the prostate cancer tissue and hepatocellular carcinoma tissue, which then inhibited pyroptosis of cancer cells spontaneously. Fortunately, Drugs for pyroptosis of cancer cells have been discovered. Val-boroPro (Talabostat, PT-100), a non-selective proline-cleavable serine protease inhibitor, can stimulates the mammalian immune system and has a potential anti-cancer effect [53]. Nevertheless, a contrast also exists between pyroptosis and immune system. Okondo et al. [54] found that after Val-boroPro induced inhibition of DPP8 and DPP9, pro-caspase-1 was activated and cleaved gasdermin D to induce pyroptosis of monocytes and macrophages. However, anti-cancer mechanism of Val-boroPro still need further researches.
Pyroptosis and AIDS
Due to the high mortality rate of AIDS and difficulty to cure, AIDS is discussed separately. Doitsh et al. [55] found that caspase-1-mediated pyroptosis, which is induced by abortive HIV infection, accounts for the majority of CD4+ T cell death in lymphoid tissue. The inefficient reverse transcription of abortive HIV infection T cells results in the accumulation of viral DNA in the cytosol, which can be detected by host cells. This process can trigger caspase-1-mediated pyroptosis, and release IL-1β to induce inflammatory responses, finally forming a vicious cycle, and constantly consuming T cells of the body. This process is also accompanied by chronic inflammation, an important symptom of AIDS patients. In contrast to the massive mortality of T cell death in the lymphoid tissue, Munoz-Arias et al. [56] found that peripheral blood-derived CD4+ T cells had a natural resistance to pyroptosis. This resistance is partially due to the deeper resting state of T cells, resulting in fewer HIV-1 reverse transcription. The difference between these two researches’ conclusion highlights that lymphoid tissue microenvironment dynamically shapes the CD4+ T-lymphocytes response to HIV.
Discussion and future perspectives
The complicated mechanism of pyroptosis and its association with the internal environment have been gradually uncovered in recent years. PAMPs and DAMPs are produced along with the pathological process of infectious disease and immune disorders, indicating that both of them have a strong correlation with pyroptosis. As for neurodegenerative disease, it is mainly caused by mass mortality of neurons, partially arising from pyroptosis, leading to nerve system dysfunction. The mass mortality of endothelial cells undergoing pyroptosis can result in decreased vascular endothelial function, which is a major cause of cardiovascular disease, intricately though. Moreover, with a better understanding of the effect of pyroptosis on cancer and AIDS, we are able to see into the causes of these incurable diseases. Nonetheless, it should not be ignored that studies in pyroptosis have only been carried out in a limited region. For example, enterovirus 71 is able to escape from immune defense mechanism of the host cell through destroying gasdermin D. It is intriguing whether there exists any possibility to restrain unnecessary pyroptosis in the body by taking advantage of the characteristics of enterovirus 71. Also, it is feasible to develop targeted medicine to prevent pyroptosis, which may contribute to the treatment of neurodegenerative disease or cardiovascular disease. However, given the characteristics of AIDS and cancer, pyroptosis may be a blind alley for the development of these certain targeted drugs. The way in which we can get a breakthrough in this area remains an issue of utmost importance and requires earnest handling.
Acknowledgements
This work was supported by the Chinese National Science Foundation (31371373, 31771572), the Nature Science Foundation of Jiangsu Province (BK20151395), the Open Fund of State Key Laboratory of Natural Medicines (No. SKLNMKF201811), and the Fundamental Research Funds for the Central Universities (021414380330). Six Talent Peaks Project in Jiangsu Province (YY-014). This work was also supported by Liver Disease Collaborative Research Platform, Medical School of Nanjing University and Translational Medicine Core Facilities, Medical School of Nanjing University.
Disclosure of conflict of interest
None.
References
- 1.Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. 2017;18:127–136. doi: 10.1038/nrm.2016.149. [DOI] [PubMed] [Google Scholar]
- 2.Vande Walle L, Lamkanfi M. Pyroptosis. Curr Biol. 2016;26:R568–R572. doi: 10.1016/j.cub.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16:7–21. doi: 10.1038/nri.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rathinam VA, Fitzgerald KA. Inflammasome complexes: emerging mechanisms and effector functions. Cell. 2016;165:792–800. doi: 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi JJ, Zhao Y, Wang K, Shi XY, Wang Y, Huang HW, Zhuang YH, Cai T, Wang FC, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 7.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 8.Walsh JG, Muruve DA, Power C. Inflammasomes in the CNS. Nat Rev Neurosci. 2014;15:84–97. doi: 10.1038/nrn3638. [DOI] [PubMed] [Google Scholar]
- 9.Widmer RJ, Lerman A. Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract. 2014;2014:291–308. doi: 10.5339/gcsp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slim J, Saling CF. A review of management of inflammation in the hiv population. Biomed Res Int. 2016;2016:3420638. doi: 10.1155/2016/3420638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levinsohn JL, Newman ZL, Hellmich KA, Fattah R, Getz MA, Liu S, Sastalla I, Leppla SH, Moayeri M. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 2012;8:e1002638. doi: 10.1371/journal.ppat.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellmich KA, Levinsohn JL, Fattah R, Newman ZL, Maier N, Sastalla I, Liu SH, Leppla SH, Moayeri M. Anthrax lethal factor cleaves mouse Nlrp1b in both toxin-sensitive and toxin-resistant macrophages. PLoS One. 2012;7:e49741. doi: 10.1371/journal.pone.0049741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao KC, Mogridge J. Activation of the Nlrp1b inflammasome by reduction of cytosolic ATP. Infect Immun. 2013;81:570–579. doi: 10.1128/IAI.01003-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 15.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 16.Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015;265:35–52. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Y, Zeng MY, Yang DH, Metro B, Nunez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–7. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Nat Acad Sci U S A. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–U356. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–U516. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aubert DF, Xu H, Yang JL, Shi XY, Gao WQ, Li L, Bisaro F, Chen S, Valvano MA, Shao F. A burkholderia type VI effector deamidates Rho GTPases to activate the pyrin inflammasome and trigger inflammation. Cell Host Microbe. 2016;19:664–674. doi: 10.1016/j.chom.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Xu H, Yang JL, Gao WQ, Li L, Li P, Zhang L, Gong YN, Peng XL, Xi JZJ, Chen S, Wang FC, Shao F. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237–41. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 23.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 24.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 25.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, He WT, Hu LC, Li JX, Fang Y, Wang X, Xu XZ, Wang Z, Huang K, Han JH. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007–1020. doi: 10.1038/cr.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Muller DJ, Broz P, Hiller S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Zhang ZB, Ruan JB, Pan YD, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–8. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He WT, Wan HQ, Hu LC, Chen PD, Wang X, Huang Z, Yang ZH, Zhong CQ, Han JH. Gasdermin D is an executor of pyroptosis and required for interleukin-1 beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conti B, Park LC, Calingasan NY, Kim Y, Kim H, Bae Y, Gibson GE, Joh TH. Cultures of astrocytes and microglia express interleukin 18. Mol Brain Res. 1999;67:46–52. doi: 10.1016/s0169-328x(99)00034-0. [DOI] [PubMed] [Google Scholar]
- 31.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi JJ, Zhao Y, Wang YP, Gao WQ, Ding JJ, Li P, Hu LY, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–92. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 33.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 34.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu JS, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang YF, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 35.Yang DH, He Y, Munoz-Planillo R, Liu Q, Nunez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 37.Hanamsagar R, Aldrich A, Kielian T. Critical role for the AIM2 inflammasome during acute CNS bacterial infection. J Neurochem. 2014;129:704–711. doi: 10.1111/jnc.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pereira MS, Morgantetti GF, Massis LM, Horta CV, Hori JI, Zamboni DS. Activation of NLRC4 by flagellated bacteria triggers caspase-1-dependent and -independent responses to restrict legionella pneumophila replication in macrophages and in vivo. J Immunol. 2011;187:6447–6455. doi: 10.4049/jimmunol.1003784. [DOI] [PubMed] [Google Scholar]
- 39.Lei XB, Zhang ZZ, Xiao X, Qi JL, He B, Wang JW. Enterovirus 71 inhibits pyroptosis through cleavage of gasdermin D. J Virol. 2017;91 doi: 10.1128/JVI.01069-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palacios-Macapagal D, Connor J, Mustelin T, Ramalingam TR, Wynn TA, Davidson TS. Cutting edge: eosinophils undergo caspase-1-mediated pyroptosis in response to necrotic liver cells. J Immunol. 2017;199:847–853. doi: 10.4049/jimmunol.1601162. [DOI] [PubMed] [Google Scholar]
- 41.Maltez VI, Tubbs AL, Cook KD, Aachoui Y, Falcone EL, Holland SM, Whitmire JK, Miao EA. Inflammasomes coordinate pyroptosis and natural killer cell cytotoxicity to clear infection by a ubiquitous environmental bacterium. Immunity. 2015;43:987–997. doi: 10.1016/j.immuni.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuemmerle-Deschner JB. CAPS-pathogenesis, presentation and treatment of an autoinflammatory disease. Semin Immunopathol. 2015;37:377–385. doi: 10.1007/s00281-015-0491-7. [DOI] [PubMed] [Google Scholar]
- 43.Mortimer L, Moreau F, MacDonald JA, Chadee K. NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. Nat Immunol. 2016;17:1176–86. doi: 10.1038/ni.3538. [DOI] [PubMed] [Google Scholar]
- 44.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–8. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y, Lu M, Du RH, Qiao C, Jiang CY, Zhang KZ, Ding JH, Hu G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol Neurodegener. 2016;11:28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin Y, Li XY, Sha XJ, Xi H, Li YF, Shao Y, Mai JT, Virtue A, Lopez-Pastrana J, Meng S, Tilley DG, Monroy MA, Choi ET, Thomas CJ, Jiang XH, Wang H, Yang XF. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler Thromb Vasc Biol. 2015;35:804–816. doi: 10.1161/ATVBAHA.115.305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Li X, Pitzer AL, Chen Y, Wang L, Li PL. Coronary endothelial dysfunction induced by nucleotide oligomerization domain-like receptor protein with pyrin domain containing 3 inflammasome activation during hypercholesterolemia: beyond inflammation. Antioxid Redox Signal. 2015;22:1084–1096. doi: 10.1089/ars.2014.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure: a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24:1–52. [PubMed] [Google Scholar]
- 49.Chen HY, Lu YH, Cao ZW, Ma QL, Pi HF, Fang YL, Yu ZP, Hu HX, Zhou Z. Cadmium induces NLRP3 inflammasome-dependent pyroptosis in vascular endothelial cells. Toxicol Lett. 2016;246:7–16. doi: 10.1016/j.toxlet.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 50.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 51.Winter RN, Kramer A, Borkowski A, Kyprianou N. Loss of caspase-1 and caspase-3 protein expression in human prostate cancer. Cancer Res. 2001;61:1227–1232. [PubMed] [Google Scholar]
- 52.Chu Q, Jiang YN, Zhang W, Xu CQ, Du WJ, Tuguzbaeva G, Qin Y, Li AQ, Zhang LS, Sun GY, Cai YQ, Feng Q, Li GY, Li YY, Du ZM, Bai YL, Yang BF. Pyroptosis is involved in the pathogenesis of human hepatocellular carcinoma. Oncotarget. 2016;7:84658–84665. doi: 10.18632/oncotarget.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walsh MP, Duncan B, Larabee S, Krauss A, Davis JP, Cui Y, Kim SY, Guimond M, Bachovchin W, Fry TJ. Val-BoroPro accelerates T cell priming via modulation of dendritic cell trafficking resulting in complete regression of established murine tumors. PLoS One. 2013;8:e58860. doi: 10.1371/journal.pone.0058860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okondo MC, Johnson DC, Sridharan R, Bin Go E, Chui AJ, Wang MS, Poplawski SE, Wu WG, Liu YX, Lai JH, Sanford DG, Arciprete MO, Golub TR, Bachovchin WW, Bachovchin DA. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat Chem Biol. 2017;13:46–53. doi: 10.1038/nchembio.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doitsh G, Galloway NL, Geng X, Yang ZY, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, Greene WC. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–14. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munoz-Arias I, Doitsh G, Yang Z, Sowinski S, Ruelas D, Greene WC. Blood-derived CD4 T cells naturally resist pyroptosis during abortive HIV-1 infection. Cell Host Microbe. 2015;18:463–470. doi: 10.1016/j.chom.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


