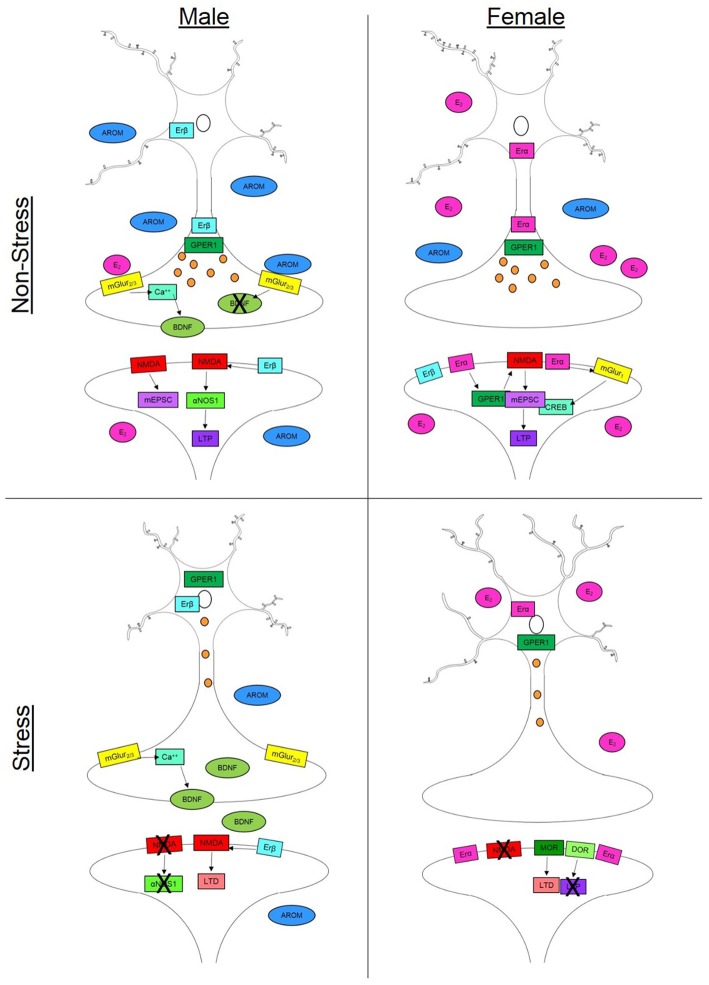
Figure 1.
Synaptic plasticity is driven by a variety of sex-specific signaling mechanisms in males and females that can vary throughout the brain. In non-stress conditions, females (top right) have increased spine density compared to males in the hippocampus (top left) but decreased dendritic length in the prefrontal cortex (PFC). This increase in spine density in the female hippocampus can occur via multiple estradiol (E2)-dependent signaling mechanisms. Binding with estrogen receptor alpha (Erα) or the G-protein coupled estrogen receptor (GPER) can initiateN-methyl-D-aspartate (NMDA) channel signaling increasing mini excitatory postsynaptic currents (mEPCS) which ultimately drive long term potentiation (LTP). Estradiol can also act through Erα on metabotropic glutamate receptor 1 (mGluR1) which in turn drives cAMP response element-binding protein (CREB) phosphorylation in females. In males, NMDA activity is driven by activation of estrogen receptor beta (Erβ). This signaling cascade includes α nitric oxide synthase-1 (αNOS1) which drives LTP in males but not females. In the male presynaptic neuron, E2-dependent activation of mGluR2/3 initiates a calcium (Ca++) signaling cascade which facilitates the release of brain derived neurotrophic factor (BDNF). Testosterone (AROM) can alter the release of BDNF and other aspects of synaptic plasticity in the baso lateral amygdala. In stress conditions, males (bottom left) show decreased dendritic branching in the PFC but slight increases in spine density in the hippocampus. Possibly accounting for this increase is the reduction in circulating steroid hormones which can allow for increased BDNF release in males. Females (bottom right) on the other hand, experience increased dendritic length in the PFC and hippocampus as well as a suppression of spine growth in the hippocampus following stress. Females exhibit changes in opiate receptor (OR) signaling that can drive long term depression (LTD)—specifically through mu- and delta-OR activity. In the presynaptic neuron, axonal labeling of Erα and GPER1 facilitates vesicle transmission down the axon in both males and females. However, in stress conditions (bottom panels), both receptors migrate to the nucleus thereby reducing vesicle trafficking.