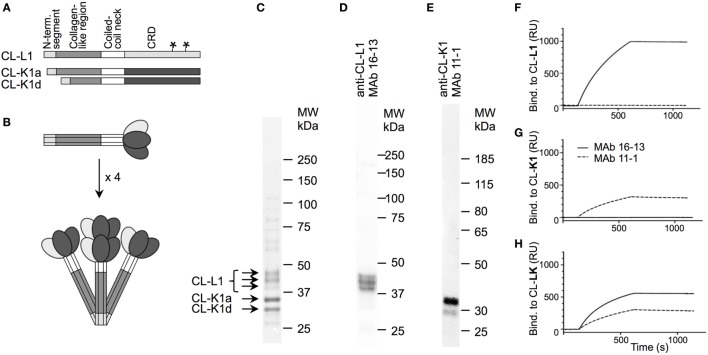
Figure 1.
Structure of CL-LK and antibody specificity. (A) Schematic illustration of the domain organization of CL-L1 and CL-K1 polypeptide chains. The symbol “*”on the CL-L1 polypeptide chain represent two N-linked glycosylation sites in the carbohydrate recognition domain. CL-K1 is found in the circulation in the form of two isoforms: CL-K1a represents full-length and CL-K1d represents an alternative spliced form devoid of a part of the collagen-like region. (B) Subunit of CL-LK and oligomeric structures. A total of three polypeptide chains of CL-K1 and CL-L1 join to form a heteromeric subunit, which may further oligomerize into structures ranging from dimers to hexamers of subunits, here illustrated by a tetramer. (C) Analysis of purified CL-LK by SDS-PAGE and visualization by silver staining. The three bands of CL-L1 represent non-, partially, and fully glycosylated forms of CL-L1. (D,E) Specificity of monoclonal antibodies by Western blotting of serum under. reducing conditions and visualization by ECL. (F–H) SPR analyses of monoclonal antibodies with immobilized CL-K1, CL-L1, and CL-LK as antigen, respectively. MAbs 16-13 (--) and 11-1 (---) were analyzed for binding to immobilized purified CL-L1 (F), CL-K1 (G), or CL-KL (H) MAb using concentrations ranging from 0.1 to 10 µg/ml.