Figure 2.
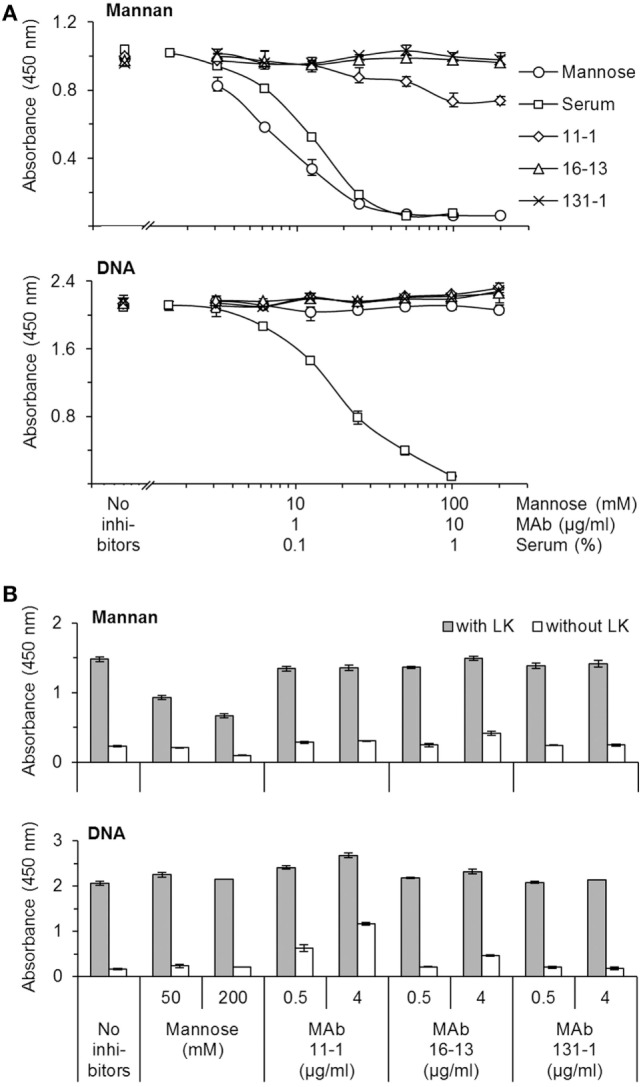
Characterization of mAbs’ impact on the ligand-binding and complement activity mediated by CL-LK. (A) Impact on ligand-binding. CL-LK was incubated on ligand-coated plates (mannan or DNA) in the presence or absence of the mAbs used for IHC (mAb 11-1 and mAb 16-13), mannose, or serum (known to interfere with the binding of CL-LK to ligands). mAb 131-1 (anti-MBL) was included as control. Bound CL-LK was detected with biotinylated mAb-anti-CL-K1 (compatible with the applied mAbs) and HRP-streptavidin. (B) Impact on CL-LK-mediated complement activation. Prior to incubation with MASP-2 and C4, plates were prepared with CL-LK and coated ligands (mannan or DNA) as above. Deposition of C4b was detected with biotin-labeled anti-C4b mAb and HRP-streptavidin. The results shown are representative of three independent experiments. Error bars refer to max and min of triplicate measurements. None of the tested mAbs interfered with ligand binding or complement activation. CL-LK binding to mannan and DNA occurs via two separate binding site, and the latter is not inhibited by mannose, whereas uncharacterized blood components inhibit both types of interaction (10, 11).
