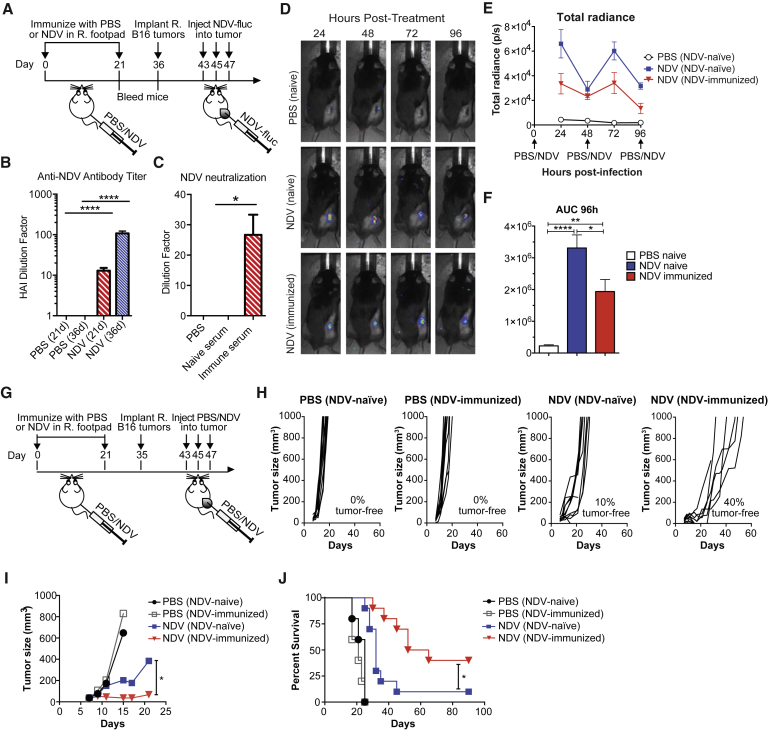
Figure 2.
Prior Immunity to NDV Potentiates Its Therapeutic Efficacy with Intratumoral Administration
(A) Prime-boost immunization scheme. Tumors were established by implantation of 2 × 105 B16-F10 cells in the right flank. (B) Anti-NDV antibody serum titers determined by hemagglutination inhibition (HI) assay at 21 and 36 days after immunization. The y axis indicates the dilution factor at which HI is no longer seen. (C) Neutralization of NDV by day 21 serum, as determined by infectivity of A549 cells. The y axis indicates the dilution factor at which NDV neutralization is no longer seen. (D) Representative luminescence images from animals treated with NDV-fluc. (E) Quantification of average luminescence from the tumor sites 24, 48, 72, and 96 hr after initial treatment. (F) AUC calculated from the data in (D). (G) Survival experiment treatment scheme. Tumors were established by implantation of 2 × 105 B16-F10 cells in the right flank. (H and I) Growth of individual injected tumors (H) and average B16-F10 tumor growth until first death in each group (I). (J) Overall survival of the treated B16-F10 tumor-bearing mice. Data for (D)–(F) represent one of two experiments with n = 5 per group. Data for (G)–(J) show representative results from one of four experiments with n = 10 per group. Mean ± SEM is shown. ns, not significant; *p < 0.05; **p < 0.01; ****p < 0.0001. R, right.