Figure 1. Impact of sepsis on carbohydrate metabolism.
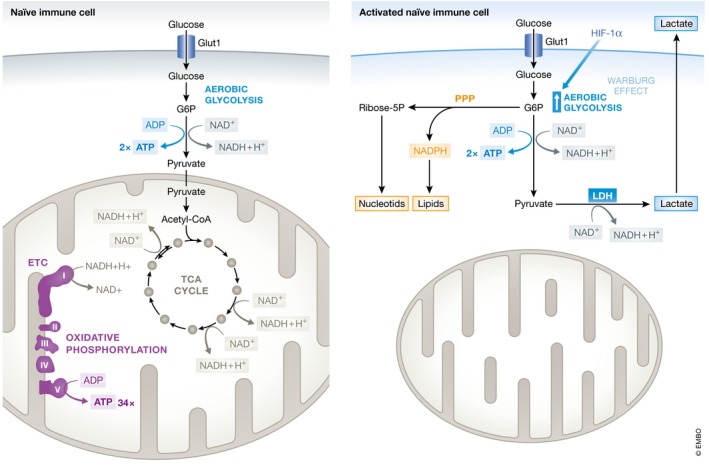
Naïve immune cells rely on glycolysis and oxidative phosphorylation as the main metabolic pathways to generate ATP. Glycolysis is the catabolic process in which glucose is converted into pyruvate. Extracellular glucose is imported into cells by GLUT1 (SLC2A1 gene). Subsequent intracellular processing of glucose yields two pyruvate molecules and two ATP molecules by a series of enzymatic reactions. At sufficient O2 tension, pyruvate is imported in mitochondria and converted into acetyl‐coA and enters the tricarboxylic acid cycle (TCA cycle, a.k.a. Kreb's cycle), after which each pyruvate yields 17 ATP molecules by the electron transport chain (ETC) and oxidative phosphorylation. Upon activation of immune cells, the glycolysis pathway is upregulated under the control of HIF‐1α. The metabolic pathway shifts from oxidative phosphorylation to aerobic glycolysis, also referred to as the Warburg effect, to meet the increased energy demand in activated immune cells. Despite the presence of abundant O2 in the environment, glucose is then directly metabolized into lactate. Although energetically less favorable (glycolysis generates 2 molecules of ATP out of 1 molecule glucose, whereas oxidative phosphorylation provides 36 ATP molecules), aerobic glycolysis allows higher velocity of glycolysis and thus faster ATP production, as well as provides important precursors for the synthesis of lipids, amino acids, and nucleotides required for proliferation. Interfering with aerobic glycolysis in immune cells has been shown to undermine their anti‐infectious activities, highlighting the importance of this altered metabolism in activated immune cells.
