Summary
Herbivore communities are shaped by indirect plant‐mediated interactions whose outcomes are strongly dependent on the sequence of herbivore arrival. However, the mechanisms underlying sequence specificity are poorly understood.
We examined the mechanisms that govern sequence‐specific effects of the interaction between two specialist maize herbivores, the leaf feeder Spodoptera frugiperda and the root feeder Diabrotica virgifera virgifera. In the field, S. frugiperda reduces D. v. virgifera abundance, but only when it arrives on the plant first.
In behavioral experiments, D. v. virgifera larvae continued feeding on plants that they had infested before leaf infestation, but refused to initiate feeding on plants that were infested by S. frugiperda before their arrival. Changes in root‐emitted volatiles were sufficient to elicit this sequence‐specific behavior. Root volatile and headspace mixing experiments showed that early‐arriving D. v. virgifera larvae suppressed S. frugiperda‐induced volatile repellents, which led to the maintenance of host attractiveness to D. v. virgifera.
Our study provides a physiological and behavioral mechanism for sequence specificity in plant‐mediated interactions and suggests that physiological canalization of behaviorally active metabolites can drive sequence specificity and result in strongly diverging herbivore distribution patterns.
Keywords: aboveground−belowground interactions, Diabrotica virgifera virgifera, induced resistance, physiological canalization, plant–herbivore interactions, Spodoptera frugiperda, volatile organic compounds, Zea mays
Introduction
Interspecific competition influences the structure, function and stability of natural and agricultural ecosystems (Loreau & de Mazancourt, 2013). For herbivorous insects, interspecific competition can occur through direct interference or through plant‐mediated, indirect effects (Denno et al., 1995). A growing number of studies show that plant‐mediated, indirect effects are the most common form of interspecific competition between herbivores (Ohgushi, 2005; Kaplan & Denno, 2007; Xiao et al., 2012; Huang et al., 2013) and that they act as driving forces of herbivore community composition in nature (Kaplan & Denno, 2007; Poelman & Dicke, 2014; Stam et al., 2014).
The outcome of plant‐mediated interactions between herbivores is determined by a number of factors, including the identity of the attacking herbivore, the identity of the plant and the identity of the responding herbivore (Johnson & Agrawal, 2005; Wurst & van der Putten, 2007; Xiao et al., 2012; Huang et al., 2014). Recently, the sequence of arrival was also identified as an important factor: depending on which species arrives first, the effect of one herbivore on the other can change drastically. Soler et al. (2012), for instance, observed that Pieris brassicae caterpillars grew bigger when feeding on Brassica oleracea plants if the plants were infested by Brevicoryne brassicae aphids before the arrival of P. brassicae, but not if both herbivores attacked the plant simultaneously. A recent meta‐analysis of interactions between leaf‐ and root‐feeding herbivores identified the sequence of arrival as a strong predictor for the directionality of effects for this type of plant‐mediated interaction (Johnson et al., 2012).
To date, several physiological hypotheses have been proposed that may explain sequence specificity (Erb et al., 2011a; Stam et al., 2014): plant‐mediated feedback loops, overriding induction effects and physiological canalization. Plant‐mediated feedback loops occur if two herbivores sharing a host plant influence each other reciprocally (Soler et al., 2012): a first arriving herbivore could then influence the behavior and damage patterns of a second arriver by inducing physiological changes in the plant, which, as a consequence, would change the plant‐mediated impact of the second herbivore on the first herbivore and thereby lead to sequence‐specific patterns. Overriding effects occur if one herbivore elicits a plant response that is much stronger than the response elicited by the other herbivore and thereby determines the resulting interaction (Stam et al., 2014). Physiological canalization is a phenomenon where plant responses are determined by the first arriving herbivore (Viswanathan et al., 2007). By suppressing the response that is normally elicited by a second herbivore, physiological canalization can lead to sequence‐specific effects.
Behavioral mechanisms may also lead to sequence specificity (Erb et al., 2011a; Karban, 2011). Asymmetrical host acceptance, for instance, refers to situations where a herbivore is less likely to start feeding on a new host plant than to continue feeding on a colonized host. This is a common pattern for sedentary herbivores such as miners and gall feeders and may lead to sequence‐specific effects by modulating the behavior of a herbivore differently, depending on whether it is arriving on a host plant second or whether it is already established when another herbivore arrives.
Plant physiological and herbivore behavioral mechanisms are not mutually exclusive. Asymmetrical host acceptance, for instance, may be favored by plant‐mediated feedback loops, overriding effects or physiological canalization. For example, a first arriving herbivore may negatively impact a second herbivore, which may decrease the capability of the second herbivore to induce volatile repellents, and in turn render the plant more attractive to the first herbivore. Furthermore, a first herbivore may trigger strong physiological changes in the plant which may render it attractive to itself irrespective of the potentially unattractive changes that are induced by a second arriving herbivore. Finally, a first herbivore may change the plant's physiology in a way that makes it unresponsive to the second herbivore, which may lead to the suppression of an otherwise unattractive physiological change. To date, the contributions of the different physiological and behavioral mechanisms and their combinations to sequence specificity have not been tested experimentally. As a consequence, the drivers of sequence specificity in indirect, plant‐mediated interactions are not well understood.
Here, we analyzed potential mechanisms leading to sequence specificity by studying the effect of attack by the leaf‐feeding larvae of Spodoptera frugiperda on the root‐feeding larvae of Diabrotica virgifera virgifera sharing maize (Zea mays) as a common host plant. Both herbivores occur on cultivated maize and its wild ancestors and cause severe damage in both agricultural and natural systems (Branson & Krysan, 1981; O'Day, 1998). They overlap spatially and temporally in the field, with their sequence of arrival varying considerably with climatic conditions and locations (Branson, 1976; O'Day, 1998). Our previous study within the same system revealed that S. frugiperda larvae significantly reduce the number of D. v. virgifera larvae feeding on maize roots in the field, but only when S. frugiperda larvae arrive first (Erb et al., 2011a). Subsequent experiments showed that maize root systems of plants which are attacked by leaf‐feeding caterpillars become highly unattractive to D. v. virgifera larvae, and that this effect is mediated by long‐ and short‐distance host acceptance cues (Robert et al., 2012a; Erb et al., 2015; Lu et al., 2016). By contrast, D. v. virgifera attack renders the plant highly attractive to conspecifics (Robert et al., 2012a) and reprograms the root metabolism to become more suitable for its own development (Robert et al., 2012b). Although D. v. virgifera reduces the performance of leaf‐feeders on maize under water‐limiting conditions, which may lead to plant‐mediated feedback loops (Erb et al., 2009, 2011b), we found no correlation between the amount of S. frugiperda leaf damage and the reduction of D. v. virgifera performance in our previous work (Erb et al., 2011a).
Based on these findings, we hypothesized that asymmetrical host acceptance may contribute to the sequence‐specific interaction patterns between D. v. virgifera and S. frugiperda, and that this asymmetrical acceptance behavior may be the result of either overriding effects or physiological canalization. We therefore conducted a series of behavioral experiments to explore the impact of the sequence of arrival on host plant attractiveness and acceptability for D. v. virgifera larvae. We then used a modified two‐by‐two‐arm olfactometer to test the influence of plant volatiles on the sequence‐specific behavior of D. v. virgifera and to distinguish between overriding effects and physiological canalization. Finally, we analyzed the changes in root volatiles elicited by the different arrival sequences to test for patterns of physiological canalization.
Materials and Methods
Plants and insects
Maize (Zea mays L.) seeds (hybrid Delprim) were obtained from Delley Seeds and Plants Ltd (Delley, Switzerland). They were sown individually in plastic pots (11 cm depth and 4 cm diameter) and placed in a glasshouse (26 ± 2°C; 14 : 10 h, light : dark; 55% relative humidity). Twelve days later (henceforth called day 0), plants with three fully developed leaves were used for experiments. Eggs of Diabrotica virgifera virgifera (LeConte) were obtained from the Agricultural Research Service, United States Department of Agriculture (Brookings, SD, USA) and larvae were reared on freshly germinated maize plants until use. Spodoptera frugiperda (J.E. Smith) eggs were obtained from the University of Neuchatel (Neuchâtel, Switzerland), and the hatching larvae were reared on a soy‐wheat germ diet (Bio‐Serv, Flemington, NJ, USA) until use.
Plant treatments
To establish different feeding sequences and herbivore combinations, plants were randomly assigned to one of four treatments (Fig. 1a): (1) aboveground herbivory (AG): 12 second‐instar S. frugiperda larvae were added to the leaves of each plant at day 2; (2) belowground herbivory (BG): six second‐instar D. v. virgifera larvae were added into a hole (9 cm depth and 0.5 cm diameter) in the soil at the base of each plant at day 0; (3) belowground attack followed by aboveground attack (BG > AG): six second‐instar larvae of D. v. virgifera were added to each plant at day 0, and 12 second‐instar larvae of S. frugiperda were added to each plant at day 2; (4) controls without herbivory (C). These treatments simulated a situation where D. v. virgifera larvae newly arrive on plants already infested with S. frugiperda (AG) or where they can continue feeding on maize plants that are infested by conspecifics alone (BG) or by conspecifics that arrived before the arrival of the leaf feeder (BG > AG) (Fig. 1a). As D. v. virgifera larvae refuse to feed on plants that have previously been attacked by S. frugiperda (Robert et al., 2012a; Erb et al., 2015; Lu et al., 2016), an AG > BG treatment was not included in the experimental set‐ups.
Figure 1.
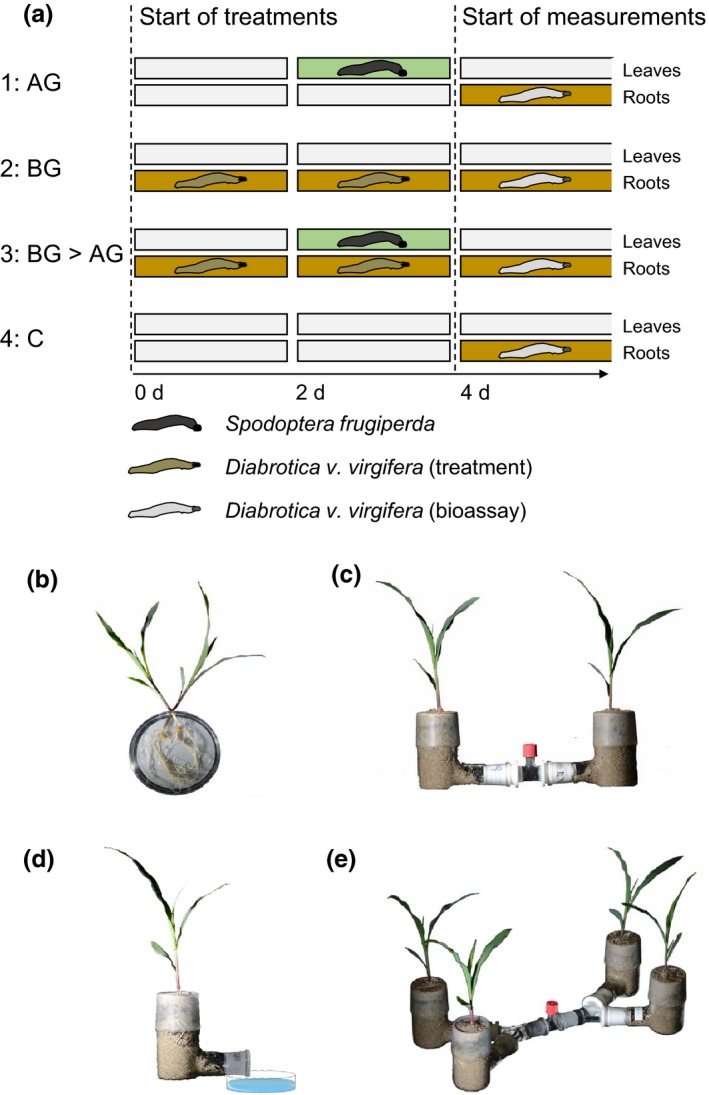
Overview of the experimental design and set‐ups used in this study. (a) Experimental treatments (infestation histories). To establish different sequences of arrival, second instar Spodoptera frugiperda larvae were added to the leaves, and second instar Diabrotica virgifera virgifera larvae were added to the roots of maize plants in different combinations. After 4 d of herbivore infestation, plants with different infestation histories were offered to D. v. virgifera larvae in choice and no‐choice experiments or were used for root volatile analyses. AG, aboveground S. frugiperda larval infestation; BG, belowground D. v. virgifera larval infestation; BG>AG, belowground infestation followed by aboveground infestation; C, control without herbivory. (b) Larval preference was measured by laying out the root systems of two plant on moist filter paper in large Petri dishes. (c) Volatile‐mediated larval preference was measured using a two‐arm belowground olfactometer. (d) Larval escape patterns were measured using a single L‐shaped glass pot and a water‐filled Petri dish to collect the escaping larvae. (e) Volatile mixing experiments were conducted using a two‐arm belowground olfactometer with two volatile sources attached to each arm of the central chamber. For more details about the different treatments and set‐ups, refer to the Materials and Methods section.
To prevent above‐ and belowground herbivores from escaping, the aboveground parts (leaves of maize plants) were caged with transparent 1.5‐l plastic bottles with their bottoms removed that were placed upside‐down on the pots. Belowground parts (pots) were covered with aluminum foil. All plants were caged in the same way regardless of herbivore treatment. Furthermore, small holes were made in the soil of each plant regardless of D. v. virgifera infestation. Four days after the beginning of the different treatments (day 4), the plastic bottles and S. frugiperda larvae were removed. Then, the responding D. v. virgifera larvae were introduced into the system (Fig. 1b–e). Timing and herbivore densities were chosen to match earlier studies and to mimic natural occurrence patterns in the field (Erb et al., 2011a; Robert et al., 2012a).
Influence of sequence of arrival on host plant acceptance by D. v. virgifera
In a first set of experiments, we tested the hypothesis that D. v. virgifera larvae may reject roots of plants that are previously infested with S. frugiperda, but may continue to feed on plants on which they were able to establish a suitable feeding environment before the arrival of the leaf‐feeder. We conducted experiments using three different set‐ups (Fig. 1b–d).
First, we tested the behaviour of D. v. virgifera using a Petri dish set‐up which allowed for direct root contact (Robert et al., 2012c) (Fig. 1b). The root systems of plants from the different treatment groups were gently washed with tap water. Plants were then paired in the following combinations: (1) C vs AG; (2) C vs BG; (3) C vs BG > AG. Root systems of the different plant pairs were placed on a moistened filter paper in a Petri dish (13.5 cm diameter and 2 cm depth), which had a gap (0.8 cm width and 2 cm height) in the side. The stems were laid into the gap, with the leaves remaining outside the Petri dish. Six second‐instar larvae were then added to the moistened filter paper. The larvae could move and feed freely on the plants within the Petri dish. The Petri dish was covered with aluminum foil to decrease the impact of light on the roots and insects. The position of the larvae was recorded at 0.5, 1.5, 3 and 5 h. Larvae that remained on the filter paper and did not choose a plant were counted as no choice. Each treatment combination was repeated 24–36 times.
Second, we specifically tested the contribution of volatile cues to the observed behavioral patterns. For this purpose, the same treatment combinations as in the first experiment were offered to D. v. virgifera larvae in two‐arm olfactometers as described previously (Robert et al., 2012a) (Fig. 1c). Before the beginning of the treatments, plants were transplanted individually into L‐shaped glass pots (11 cm depth and 5 cm diameter) with a horizontal connector at a height of 0.5 cm and filled with moist sand. At day 4, the horizontal connector of each glass pot was attached with one Teflon connector (29/32 to 24/29 mm) which contained a fine metal screen (2300 mesh; Small Parts Inc., Miami Lakes, FL, USA). Then, the two Teflon connectors were linked using a glass tube (24/29 mm; length 8 cm) with a vertical access port in the middle. To keep the root systems in the dark and to avoid visual cues for the larvae, the entire olfactometer was covered with aluminum foil. Twenty minutes after connecting the different odor sources, six second‐instar D. v. virgifera larvae were released into the access port of the glass tube. The larvae could move freely in the glass tube, but could not reach the roots of the plants. After 10 min, the olfactometer was disassembled and the number of larvae in each Teflon connector was recorded. Larvae that stayed in the central glass tube after 10 min were recorded as no choice. For each treatment combination, 18 independent replicates were carried out.
In a third experiment, we tested whether D. v. virgifera larvae are more likely to leave the rhizosphere environment of infested plants, even in the absence of an alternative host. For this purpose, plants were potted and infested in L‐shaped glass pots as described for the second experiment (Fig. 1d). Then, six second‐instar larvae were released directly at the entrance of the horizontal access port of each glass pot. The access port of the horizontal connector was not sealed so the larvae could move into the soil and start feeding or try to escape from the plant through the access port. The L‐pot was placed in a Petri dish filled with tap water at a height of 0.5 cm to catch escaping D. v. virgifera larvae without flooding the glass pot. The number of escaped larvae in the trap was recorded over 20 min. For each treatment, 12 replicates were carried out.
Plant‐mediated feedback loops
To evaluate whether belowground attack by D. v. virgifera changes the pattern of aboveground damage by S. frugiperda larvae under the current experimental conditions, the leaves of plants from the different infestation treatments were collected at day 4, and total leaf area and damaged leaf area were measured for each plant using digimizer software (MedCalc Software bvba, Mariakerke, Belgium). Eighteen replicates per treatment were carried out.
Overriding effects
To investigate whether an overriding signal may be responsible for the observed asymmetrical host acceptance of D. v. virgifera in the first set of experiments, we developed a two‐by‐two arm belowground olfactometer that allowed us to combine the volatile headspaces from two odor sources per arm (Fig. 1e). For this purpose, the two‐arm olfactometer set‐up was modified as follows. Two Teflon connectors attached to glass pots were linked using a ‘Y’ glass tube (24/29 mm; length 8 cm) at an angle of 60°. Then, two ‘Y’ glass tubes were connected to a central glass tube (24/29 mm; length 8 cm) with a vertical access port in the middle. This modification enabled us to attach two L‐shaped glass pots to each side of the release tubes and to test the preference of D. v. virgifera for two combinations of two mixed odor sources. The following treatment combinations were investigated using this set‐up: C + C vs C + AG; C+C vs C + BG, and C + C vs AG + BG. The olfactometer was disassembled and the number of larvae in each ‘Y’ glass tube was recorded after 10 min. We hypothesized that, if D. v. virgifera elicits an overriding signal, the AG + BG arms should be more attractive than the C + C arm. Eighteen replicates were performed for each treatment combination.
Physiological canalization
To evaluate whether D. v. virgifera attack canalizes the root volatile response in a way that suppresses responsiveness to S. frugiperda infestation, we collected and analyzed root volatile profiles using solid‐phase micro‐extraction−gas chromatography−mass spectrometry (SPME‐GC‐MS). Plants were treated as described in the section ‘Plant treatments’ (Fig. 1a). Crown and primary roots were then washed with tap water and frozen in liquid nitrogen. Twelve plants per treatment were harvested, and the roots of two plants were pooled for analysis, resulting in six biological replicates. The crown and primary roots of each replicate were ground into a fine powder, and 50 mg of each root type was placed in a 10‐ml glass vial and sealed using Teflon tape (polytetrafluoroethylene). An SPME fiber (100‐μm polydimethylsiloxane coating; Supelco, Bellefonte, PA, USA) was then inserted into the vial for 60 min at 50°C. The incubated fibers were then immediately analyzed by GC‐MS (Agilent 7820A GC interfaced with an Agilent 5977E MSD, Palo Alto, CA, USA) following previously established protocols with a few modifications (Erb et al., 2011c). Briefly, the fiber was inserted into the injector port at 250°C and desorbed for 2 min. After fiber insertion, the column temperature was maintained at 60°C for 1 min and then increased to 250°C at 5°C min−1 followed by a final stage of 4 min at 250°C. The overall analysis time for each sample, including oven cooling, was 45 min. Furthermore, to eliminate the impact of background peaks, three glass vials without any plant material (blanks) were run using the same protocol. The resulting GC‐MS chromatograms were processed with progenesis qi (Nonlinear Dynamics, Newcastle, UK) using default settings for spectral alignment and peak picking. From the resulting matrix, all features that were presented in more than one blank were removed, resulting in 232 features. Features were assigned to individual compounds by retention time and peak shape matching and identified using the NIST search 2.2 Mass Spectral Library (Gaithersburg, MD, USA) as well as retention time and spectral comparison with pure compounds.
Data analysis
To examine host acceptance of D. v. virgifera in a Petri dish experiment, the number of larvae found on different herbivory treatment groups was analyzed using a Wald test applied to a generalized linear mixed model (GLMM) with a Poisson distribution. We considered plant treatment as a fixed factor, time as a covariate and the replicate as a random factor. Each plant combination (C vs AG, C vs BG and C vs BG > AG) was analyzed separately. Then, to compare the preference of D. v. virgifera between the different treatment groups, the number of larvae on infested plants (AG, BG and BG > AG) was analyzed using a likelihood ratio test applied to a generalized linear model (GLM) with a Poisson distribution. The models included herbivory as a fixed factor and time as a covariate. The preference of D. v. virgifera larvae in the olfactometer experiments and the number of escaped larvae in the escape experiment were analyzed in the same manner. To examine whether belowground attack by D. v. virgifera larvae changes the pattern of aboveground damage by S. frugiperda larvae, the relative and absolute leaf damage of S. frugiperda larvae was analyzed using independent sample t‐tests (BG vs BG > AG). The absolute leaf damage was estimated from the sum of leaf damaged area for each plant and the relative leaf damage was calculated as the sum of leaf damaged area/the sum of total leaf area × 100 for each plant. To examine the overall differences in volatile profiles, the relative abundance of the detected features was subjected to redundancy analysis (RDA) using the different treatments as a unique explanatory variable. Monte Carlo tests with 999 permutations were then used to test for significant differences between treatments. For more detailed, compound‐specific analyses, the different features were assigned to individual compounds, and the relative abundances of the individual compounds, which corresponds to the sum of the signal intensities of the individual features, were analyzed by one‐way ANOVAs followed by least square mean post hoc tests for pairwise comparisons, including false discovery rate (FDR) corrections (Benjamini & Hochberg, 1995). All analyses were conducted using R 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria) with ‘car’, ‘lme4’, ‘lsmeans’, ‘vegan’ and ‘RVAideMemoire’ packages (Fox & Weisberg, 2011; Bates et al., 2015; Hervé, 2016; Lenth, 2016; Oksanen et al., 2016).
Results
Diabrotica virgifera virgifera rejects S. frugiperda‐infested plants only when arriving second
In the Petri dish experiment, D. v. virgifera larvae strongly preferred the roots of control plants when offered uninfested vs leaf‐infested plants (χ2 = 30.753; P < 0.001; Fig. 2a). By contrast, the larvae showed a strong preference for roots that had previously been infested with D. v. virgifera larvae over controls (χ2 = 69.919; P < 0.001; Fig. 2b). Roots that were infested with D. v. virgifera 2 d before the onset of S. frugiperda attack remained highly attractive (χ2 = 21.734; P < 0.001; Fig. 2c). The number of responding D. v. virgifera larvae increased with experimental time (C vs AG: χ2 = 5.698; P = 0.017; C vs BG: χ2 = 20.033; P < 0.001; C vs BG > AG: χ2 = 35.964; P < 0.001; Fig. 2). At the end of the experiment, 65%, 67% and 70% of D. v. virgifera larvae made a choice in C vs AG, C vs BG and C vs BG > AG, respectively. No significant interactive effects between time and treatment were found (C vs AG: χ2 = 3.515; P = 0.061; C vs BG: χ2 = 0.135; P = 0.713; C vs BG > AG: χ2 = 1.342; P = 0.247). Overall, more D. v. virgifera larvae fed on BG and BG > AG roots than on AG roots (χ2 = 38.558; P < 0.001; Fig. 2). No difference was found between the preference of D. v. virgifera for BG and BG > AG roots (P = 0.064; Fig. 2).
Figure 2.
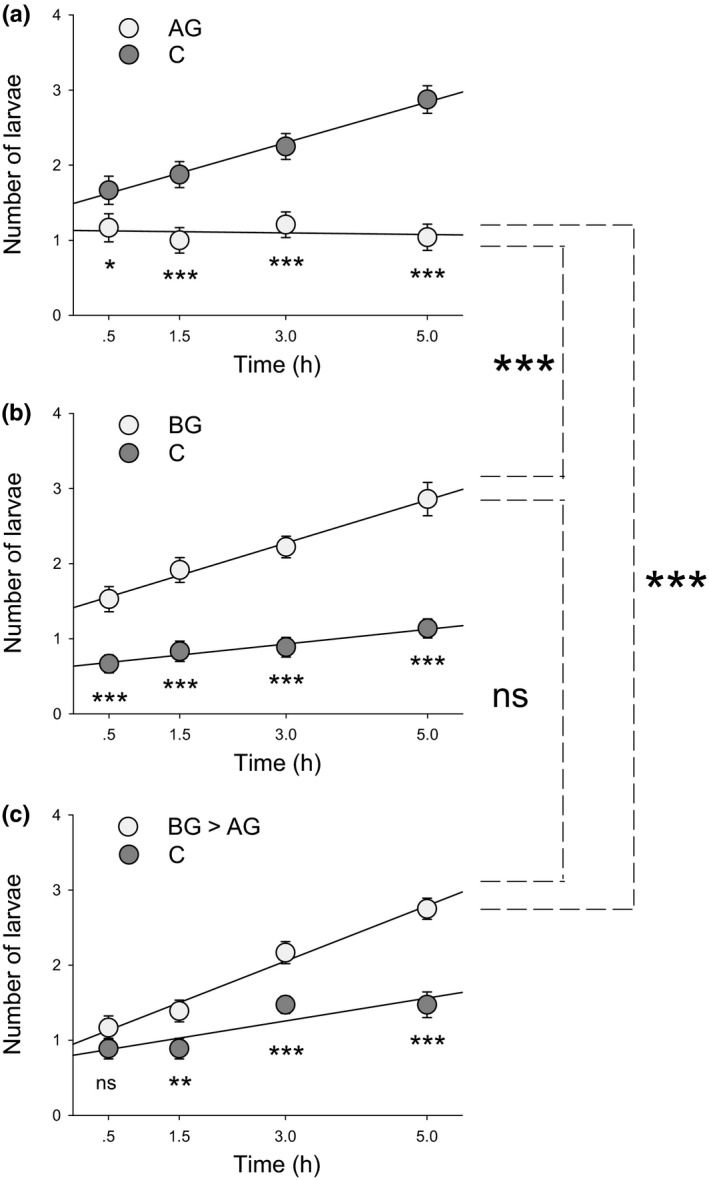
Sequence of arrival determines root attractiveness to Diabrotica virgifera virgifera. The number of D. v. virgifera larvae on the roots of plants with different infestation histories was measured in a Petri dish experiment. (a) Diabrotica virgifera virgifera choice between C and AG plants (n = 24). (b) Diabrotica virgifera virgifera choice between C and BG plants (n = 36). (c) Diabrotica virgifera virgifera choice between C and BG > AG plants (n = 36). AG, aboveground Spodoptera frugiperda larval infestation; BG, belowground D. v. virgifera larval infestation; BG > AG, belowground infestation followed by aboveground infestation; C, control without herbivory. Values correspond to mean ± 1 SE. Asterisks indicate a significant difference in preference within each combination and time‐point (ns, nonsignificant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; GLMM). Differences in preference patterns between treatment combinations are depicted by dashed lines and asterisks on the right of the graph (ns, nonsignificant; ***, P < 0.001; GLM).
In the two‐arm olfactometer experiment, similar preference patterns were observed. Diabrotica virgifera virgifera larvae showed a strong preference for control plants over S. frugiperda‐infested plants (χ2 = 8.111; P < 0.01; Fig. 3). By contrast, the larvae preferred plants that were previously infested with conspecifics over controls (χ2 = 34.177; P < 0.001; Fig. 3). Plants infested with D. v. virgifera before S. frugiperda infestation remained highly attractive (χ2 = 16.849; P < 0.001; Fig. 3). In this experiment, all larvae made a choice within 10 min. Overall, the larvae were more attracted to the roots that had been infested by conspecifics alone and conspecifics that had arrived before the arrival of the S. frugiperda, while they were less attracted to the roots that had been infested by S. frugiperda alone (χ2 = 20.396; P < 0.001; Fig. 3). Again, BG and AG > BG treatments were not significantly different from each other (P = 0.389; Fig. 3).
Figure 3.
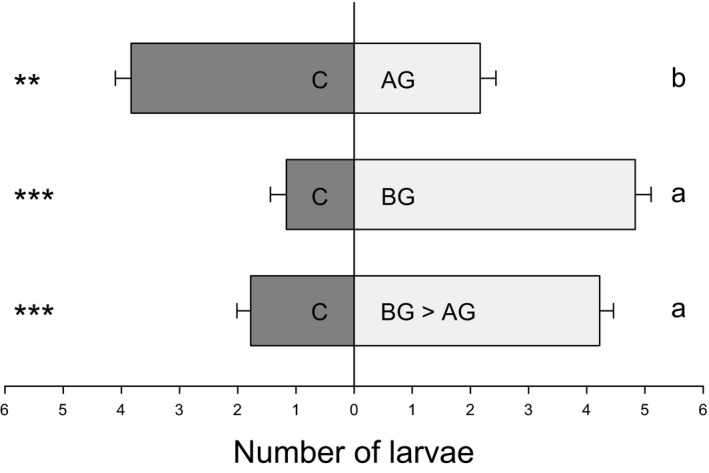
Volatile cues contribute to sequence‐specific preference patterns of Diabrotica virgifera virgifera. The number of D. v. virgifera larvae attracted to root volatiles of plants with different infestation histories was measured in a two‐arm olfactometer experiment. AG, aboveground Spodoptera frugiperda larval infestation; BG, belowground D. v. virgifera larval infestation; BG > AG, belowground infestation followed by aboveground infestation; C, control without herbivory. Values are mean ± 1 SE (n = 18). Asterisks indicate a significant preference within each treatment combination (**, P < 0.01; ***, P < 0.001; GLMM). Different letters indicate significant differences between treatment combinations (P < 0.05; GLM).
When offered a single host plant, the number of escaping D. v. virgifera larvae differed significantly between treatments (χ2 = 32.112; P < 0.001; Fig. 4). When offered a S. frugiperda‐infested plant, 50% of the larvae escaped from the rhizosphere within 20 min (Fig. 4). By contrast, < 18% of the larvae left the soil of control plants or plants that were previously infested with conspecifics (Fig. 4). A similar percentage of larvae chose to remain in the rhizosphere of plants that were infested with D. v. virgifera before S. frugiperda attack (Fig. 4).
Figure 4.
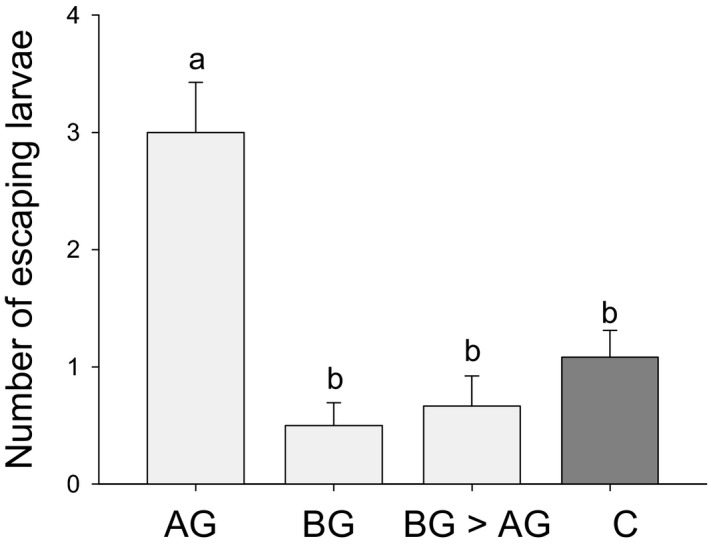
Stay‐or‐leave patterns of Diabrotica virgifera virgifera are determined by the sequence of arrival. The number of D. v. virgifera larvae leaving the rhizosphere of plants with different infestation histories was measured in an escaping experiment. AG, aboveground Spodoptera frugiperda larval infestation; BG, belowground D. v. virgifera larval infestation; BG > AG, belowground infestation followed by aboveground infestation; C, control without herbivory. Values are mean ± 1 SE (n = 12). Different letters indicate significant differences between treatments (P < 0.05; GLM).
Plant‐mediated feedback loops are unlikely to explain D. v. virgifera behavior
There was no significant difference in relative (t = 0.055; P = 0.957) or absolute (t = 1.236; P = 0.225) damaged leaf area between plants that were infested with D. v. virgifera and plants with roots that were herbivore free (Supporting Information Fig. S1). These results suggest that the interaction between D. v. virgifera and S. frugiperda is highly asymmetrical and that plant‐mediated feedback loops are unlikely to play a major role in determining sequence‐specific responses of D. v. virgifera.
Diabrotica virgifera virgifera does not produce an overriding attractive signal
Similarly to the two‐arm olfactometer experiment, D. v. virgifera larvae significantly more often preferred to move to the side of the olfactometer containing two control plants rather than the arm leading to a control plant and an S. frugiperda‐infested plant (χ2 = 15.446; P < 0.001; Fig. 5). The opposite was true for a combination of a control plant with a D. v. virgifera‐infested plant, which was attractive to the root feeder (χ2 = 8.111; P < 0.01; Fig. 5). In contrast to the attractiveness of BG > AG plants observed in the two‐arm olfactometer experiment, however (Fig. 3), the mixed rhizosphere volatiles from an S. frugiperda‐ and a D. v. virgifera‐infested plant were highly unattractive, and significantly more larvae moved to the control side (χ2 = 10.333; P < 0.01; Fig. 5) than to the AG + BG side. All larvae made a choice within the first 10 min. Overall, the presence of plants that were infested by S. frugiperda significantly more often repelled D. v. virgifera (χ2 = 15.915; P < 0.001; Fig. 5). This experiment falsifies the hypothesis that D. v. virgifera triggers an overriding attractant.
Figure 5.
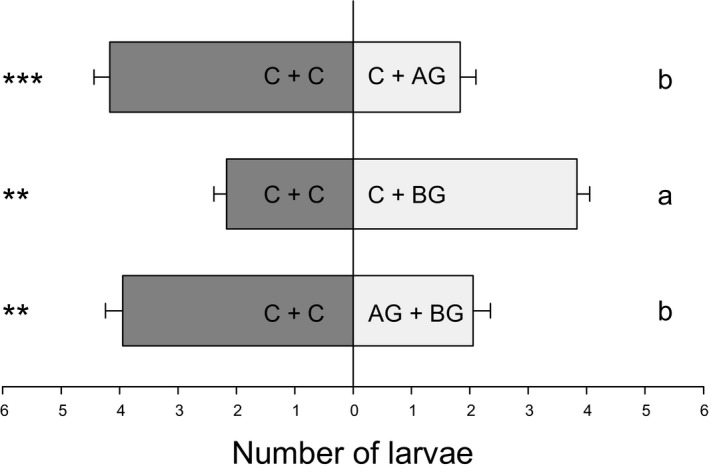
Acceptance of Diabrotica virgifera virgifera is determined by the additive changes in root volatiles. The number of D. v. virgifera larvae attracted by mixed root volatiles from plants with different infestation histories were measured in a volatile‐mixing experiment, with each arm containing two different volatile sources. AG, aboveground Spodoptera frugiperda larval infestation; BG, belowground D. v. virgifera larval infestation; C, control without herbivory. Values are mean ± 1 SE (n = 18). Asterisks indicate a significant preference within choice combinations (**, P < 0.01; ***, P < 0.001; GLMM). Different letters indicate differences in preference patterns between treatments (P < 0.05; GLM).
Diabrotica virgifera virgifera feeding suppresses S. frugiperda‐induced root volatiles
In total, we detected 232 volatile features in the GC‐MS chromatograms. Redundancy analysis revealed that S. frugiperda and D. v. virgifera attack induced different volatile blends compared with control plants and compared with each other (AG vs C: P = 0.008; BG vs C: P = 0.008; BG>AG vs C: P = 0.008; Fig. 6). The volatile profiles of plants that were induced by D. v. virgifera before S. frugiperda attack were indistinguishable from those of plants that were infested with D. v. virgifera alone (BG > AG vs BG: P = 0.642; Fig. 6), but both were significantly different from those of plants that were infested with S. frugiperda alone (BG vs AG: P = 0.008; BG > AG vs AG: P = 0.008; Fig. 6). Analysis of variance revealed 12 volatile compounds whose abundance differed significantly between treatments (Fig. 7). Pairwise comparisons showed that four of these volatiles were significantly induced by D. v. virgifera infestation alone (Fig. 7a–d) and two of them were significantly induced by S. frugiperda attack alone (Fig. 7k–l). We found no significant effect of later S. frugiperda attack on D. v. virgifera‐induced volatile emissions (Fig. 7). However, the induction of the S. frugiperda‐induced volatiles was suppressed by early D. v. virgifera infestation (Fig. 7l). This result demonstrates that D. v. virgifera canalizes the root volatile production and renders roots unresponsive to leaf attack by S. frugiperda.
Figure 6.
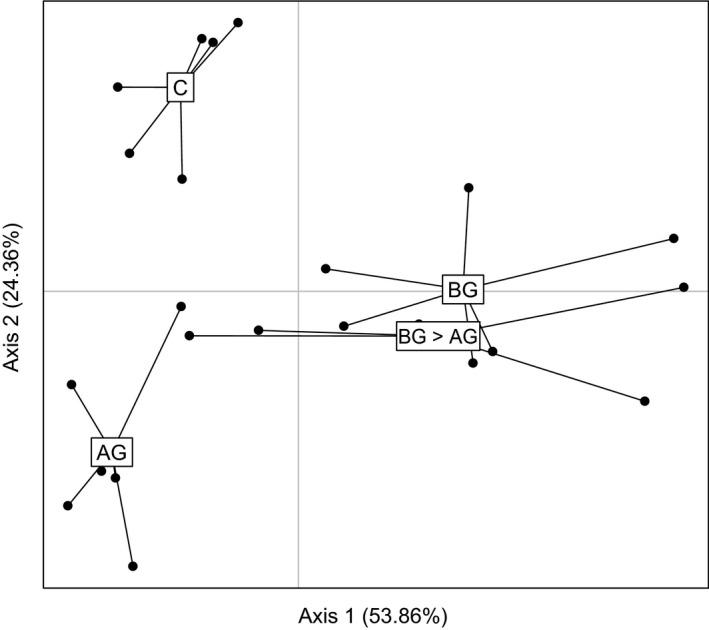
Infestation by Diabrotica virgifera virgifera canalizes the volatile response of maize roots. The results of a redundancy analysis (RDA) of the root volatile response to different sequences of D. v. virgifera and Spodoptera frugiperda feeding are shown. The first two axes explained 53.86% and 24.36% of the total variation, respectively. AG, aboveground S. frugiperda larval infestation; BG, belowground D. v. virgifera larval infestation; BG > AG, belowground infestation followed by aboveground infestation; C, control without herbivory. Data points represent individual replicates (n = 6).
Figure 7.
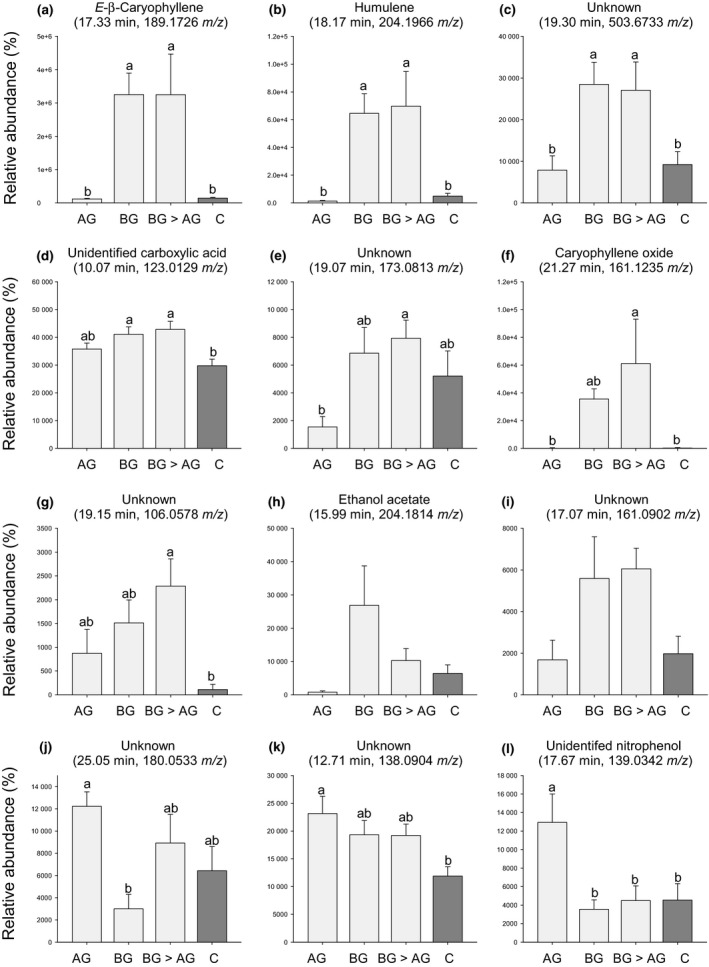
Diabrotica virgifera virgifera suppresses Spodoptera frugiperda‐induced root volatiles. The relative abundances of root volatiles in four treatments were measured using solid‐phase micro‐extraction (SPME) in combination with gas chromatograpy and mass spectrometry (GC‐MS). (a) E‐β‐Caryophyllene (17.33 min; 189.1726 m/z), (b) humulene (18.17 min; 204.1966 m/z), (c) unknown (19.30 min; 503.6733 m/z), (d) unidentified carboxylic acid (10.07 min; 123.0129 m/z), (e) unknown (19.07 min; 173.0813 m/z), (f) caryophyllene oxide (21.27 min; 161.1235 m/z), (g) unknown (19.15 min; 106.0578 m/z), (h) ethanol acetate (15.99 min; 204.1814 m/z), (i) unknown (17.07 min; 161.0902 m/z), (j) unknown (25.05 min; 180.0533 m/z), (k) unknown (12.71 min; 138.0904 m/z) and (l) unidentified nitrophenol (17.67 min; 139.0342 m/z). AG, aboveground S. frugiperda larval infestation; BG, belowground D. v. virgifera larval infestation; BG > AG, belowground infestation followed by aboveground infestation; C, control without herbivory. Values are mean ± 1 SE (n = 6). Different letters indicate differences in relative abundance among treatments (P < 0.05; LM).
Discussion
The sequence of arrival is increasingly recognized as an important determinant of plant‐mediated indirect interactions between herbivores (Viswanathan et al., 2005, 2007; Poelman et al., 2008; Erb et al., 2011a; Soler et al., 2012; Wang et al., 2014). However, the mechanisms leading to sequence specificity are not well understood. The goal of the present study was to identify the (mutually nonexclusive) behavioral and physiological mechanisms that may contribute to sequence‐specific effects. Our experiments show that leaf attack by S. frugiperda strongly reduces the attractiveness of roots for D. v. virgifera through changes in volatile cues. However, prior D. v. virgifera attack suppresses these changes and thereby maintains the attractiveness of the plants to D. v. virgifera larvae. This form of asymmetrical host acceptance behavior explains why S. frugiperda reduces the abundance of and damage by D. virgifera in the field only when arriving first on the plant (Erb et al., 2011a).
Several nonexclusive physiological mechanisms may explain why D. v. virgifera is repelled by S. frugiperda‐attacked plants only when arriving second. It is for instance possible that early‐arriving D. v. virgifera larvae change the behavior and induction pattern of S. frugiperda. However, we found no evidence for the presence of resistance feedback loops in our system: S. frugiperda damage remained unchanged by D. v. virgifera attack. Earlier studies demonstrated that D. v. virgifera root attack increases leaf resistance via ABA signaling under drought conditions; when plants were well watered, no negative effects of D. v. virgifera on Spodoptera littoralis growth were observed any more (Erb et al., 2011b). The maize seedlings in our experiments were supplied with sufficient soil moisture, which probably prevented potential feedback loops from occurring. Another explanation for the observed behavioral patterns is that D. v. virgifera may induce changes that strongly increase the attractiveness of the roots and override any negative changes that are later induced by S. frugiperda. By mixing volatiles from different plants, we tested this hypothesis on a behavioral level. Surprisingly, we found that D. v. virgifera rejected the volatile mix from a combination of plants that had been infested by D. v. virgifera and S. frugiperda separately. This is in stark contrast with the strong attractiveness of plants that were infested with D. v. virgifera and S. frugiperda sequentially and strongly suggests that D. v. virgifera does not produce an overriding attractive signal.
In contrast, our GC‐MS analyses provide clear evidence that D. v. virgifera canalizes the plant's root volatile response. Maize roots responded strongly to D. v. virgifera attack and produced higher amounts of several volatiles, including several products of the terpene synthase TPS23 which are strongly induced by D. v. virgifera (Köllner et al., 2008; Hiltpold et al., 2011) and attract the root feeder (Robert et al., 2012a). These responses were not altered by later S. frugiperda attack. By contrast, S. frugiperda attack induced a different set of compounds in the roots, including a yet unidentified nitrophenol, and this induction was fully suppressed by prior D. v. virgifera attack. These results demonstrate that early‐arriving D. v. virgifera canalizes the root metabolism in a way that makes it unresponsive to S. frugiperda attack. Canalization of plant responses by herbivores has been proposed to occur in a number of plant–herbivore interactions (Thaler et al., 2002; Viswanathan et al., 2005; Utsumi et al., 2010). For example, Viswanathan et al. (2007) found that tortoise beetle (Plagiometriona clavata) attack after flea beetle (Psylliodes affinis) attack of Solanum dulcamara did not alter the induced resistance elicited by the flea beetles. By contrast, tortoise beetle attack before flea beetle attack resulted in the disappearance of induced resistance. One possible explanation of canalization is negative cross‐talk between signaling pathways such that inducing one pathway may attenuate or repress other pathways (Koornneef & Pieterse, 2008; Erb et al., 2012). Furthermore, priority in occupying a plant resource may also result in physiological canalization, as resources invested into an initial induced response may not be available for investment into later induced responses (Stam et al., 2014). In combination with the behavioral experiments, these results suggest that the asymmetrical host acceptance behavior of D. v. virgifera is caused by physiological canalization.
In a previous study, we found that leaf attack by S. littoralis leads to a slight decrease in root ethylene production, and that adding ethylene back to the root system restores the attractiveness of the roots to D. v. virgifera (Robert et al., 2012a). Many herbivores increase local ethylene emissions of their host plants (Winz & Baldwin, 2001; von Dahl & Baldwin, 2007; Schäfer et al., 2011), and it is therefore possible that D. v. virgifera attack resulted in the reversal or canalization of the ethylene response of the roots. Unfortunately, ethylene emissions could not be measured in the current series of experiments. However, the presented findings suggest that S. frugiperda attack also triggers the release of repellent volatiles which are suppressed by D. v. virgifera. The escape experiment in particular shows that D. v. virgifera systematically moves away from leaf‐infested plants, and it seems unlikely that a reduction in ethylene concentrations alone can account for this result. Furthermore, the volatile mixing experiment suggests that the volatile blend of the roots of an S. frugiperda‐attacked plant overrides the attractive signal from a D. v. virgifera‐infested root system.
In our GC‐MS chromatograms, we found several volatiles that increased in the roots of S. frugiperda‐attacked plants. Elucidating their structure and bioactivity is an exciting prospect for future work. A recent paper identified methyl antranilate as a repellent for neonate D. v. virgifera larvae (Bernklau et al., 2016). Although methyl antranilate was not among the S. frugiperda‐induced root volatiles, it provides an interesting starting point to identify the volatiles that render S. frugiperda‐attacked plants repellent to D. v. virgifera larvae. One aspect that should be kept in mind is that root volatiles were measured by grinding root material and sampling the headspace of the ground samples by SPME. The advantages of this technique are its sensitivity and robustness. Its disadvantage is that it may result in the detection of volatile compounds that are not actually released into the rhizosphere by intact roots. Future experiments should therefore include in vivo sampling techniques to confirm the release of the newly detected volatiles into the rhizosphere (Ali et al., 2010; Hiltpold et al., 2011).
Host location and acceptance by herbivores are key processes in plant–herbivore interactions. Our results show that physiological canalization can have a strong, sequence‐specific impact on host acceptance by herbivores, which may result in strongly diverging herbivore damage and distribution patterns in the field. Our previous work shows that the repellent effect of leaf infestation on root herbivores is highly conserved across herbivore species and maize genotypes (Lu et al., 2016). Whether similar effects also occur in other plant species remains to be elucidated. Understanding the mechanisms that govern sequence specificity will allow for the integration of this phenomenon into current theory on plant‐mediated interactions and will facilitate future efforts to develop predictive ecophysiological models of multi‐herbivore dynamics on shared host plants.
Author contributions
M.E., W.H. and C.A.M.R. planned and designed the research. W.H., L.H., Z.B. and C.A.M.R. carried out experiments. W.H., M.R.H. and M.E. analyzed data. M.E. and W.H. wrote the manuscript.
Supporting information
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Infestation by D. v. virgifera does not change aboveground damage by S. frugiperda larvae.
Acknowledgements
We thank Yves Borcard (FARCE Laboratory, University of Neuchâtel) for supplying S. frugiperda eggs, Wade French and Chad Nielson (ARS‐USDA Brookings) for supplying D. v. virgifera eggs and the gardeners of the IPS for their help with plant cultivation. The study was supported by the Swiss National Science Foundation (grant nos 152613 and 153517 to M.E.).
References
- Ali JG, Alborn HT, Stelinski LL. 2010. Subterranean herbivore‐induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. Journal of Chemical Ecology 36: 361–368. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed‐effects models using lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 57: 289–300. [Google Scholar]
- Bernklau EJ, Hibbard BE, Norton AP, Bjostad LB. 2016. Methyl anthranilate as a repellent for western corn rootworm larvae (Coleoptera: Chrysomelidae). Journal of Economic Entomology. 109: 1683–1690. [DOI] [PubMed] [Google Scholar]
- Branson TF. 1976. The selection of a non‐diapausing strain of Diabrotica virgifera (Coleoptera: Chrysomelidae). Entomologia Experimentalis et Applicata 19: 148–154. [Google Scholar]
- Branson TF, Krysan JL. 1981. Feeding and oviposition behavior and life cycle strategies of Diabrotica: an evolutionary view with implications for pest management. Environmental Entomology 10: 826–831. [Google Scholar]
- von Dahl CC, Baldwin IT. 2007. Deciphering the role of ethylene in plant–herbivore interactions. Journal of Plant Growth Regulation 26: 201–209. [Google Scholar]
- Denno RF, Mcclure MS, Ott JR. 1995. Interspecific interactions in phytophagous insects: competition reexamined and resurrected. Annual Review of Entomology 40: 297–331. [Google Scholar]
- Erb M, Balmer D, De Lange ES, Von Merey G, Planchamp C, Robert CAM, Röder G, Sobhy I, Zwahlen C, Mauch‐Mani B et al 2011c. Synergies and trade‐offs between insect and pathogen resistance in maize leaves and roots. Plant, Cell & Environment 34: 1088–1103. [DOI] [PubMed] [Google Scholar]
- Erb M, Flors V, Karlen D, De Lange E, Planchamp C, D'Alessandro M, Turlings TCJ, Ton J. 2009. Signal signature of aboveground‐induced resistance upon belowground herbivory in maize. Plant Journal 59: 292–302. [DOI] [PubMed] [Google Scholar]
- Erb M, Köllner TG, Degenhardt J, Zwahlen C, Hibbard BE, Turlings TCJ. 2011b. The role of abscisic acid and water stress in root herbivore‐induced leaf resistance. New Phytologist 189: 308–320. [DOI] [PubMed] [Google Scholar]
- Erb M, Meldau S, Howe GA. 2012. Role of phytohormones in insect‐specific plant reactions. Trends in Plant Science 17: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Robert CAM, Hibbard BE, Turlings TCJ. 2011a. Sequence of arrival determines plant‐mediated interactions between herbivores. Journal of Ecology 99: 7–15. [Google Scholar]
- Erb M, Robert CAM, Marti G, Lu J, Doyen G, Villard N, Barrière Y, French BW, Wolfender JL, Turlings TCJ et al 2015. A physiological and behavioral mechanism for leaf‐herbivore induced systemic root resistance. Plant Physiology 169: 2884–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S. 2011. An R companion to applied regression. Thousand Oaks, CA, USA: Sage. [Google Scholar]
- Hervé M. 2016. RVAideMemoire: diverse basic statistical and graphical functions. R package version 0.9‐61. [WWW document] URL https://CRAN.R-project.org/package=RVAideMemoire [accessed 31 October 2016].
- Hiltpold I, Erb M, Robert CAM, Turlings TCJ. 2011. Systemic root signalling in a belowground, volatile‐mediated tritrophic interaction. Plant, Cell & Environment 34: 1267–1275. [DOI] [PubMed] [Google Scholar]
- Huang W, Siemann E, Xiao L, Yang X, Ding J. 2014. Species‐specific defence responses facilitate conspecifics and inhibit heterospecifics in above‐belowground herbivore interactions. Nature Communications 5: 4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Siemann E, Yang X, Wheeler GS, Ding J. 2013. Facilitation and inhibition: changes in plant nitrogen and secondary metabolites mediate interactions between above‐ground and below‐ground herbivores. Proceedings of the Royal Society B‐Biological Sciences 280: 20131318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MTJ, Agrawal AA. 2005. Plant genotype and environment interact to shape a diverse arthropod community on evening primrose (Oenothera biennis). Ecology 86: 874–885. [Google Scholar]
- Johnson SN, Clark KE, Hartley SE, Jones TH, McKenzie SW. 2012. Aboveground‐belowground herbivore interactions. a meta‐analysis. Ecology 93: 2208–2215. [DOI] [PubMed] [Google Scholar]
- Kaplan I, Denno RF. 2007. Interspecific interactions in phytophagous insects revisited: a quantitative assessment of competition theory. Ecology Letters 10: 977–994. [DOI] [PubMed] [Google Scholar]
- Karban R. 2011. The ecology and evolution of induced resistance against herbivores. Functional Ecology 25: 339–347. [Google Scholar]
- Köllner TG, Held M, Lenk C, Hiltpold I, Turlings TCJ, Gershenzon J, Degenhardt J. 2008. A maize (E)‐β‐Caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. The Plant Cell 20: 482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef A, Pieterse CMJ. 2008. Cross talk in defense signaling. Plant Physiology 146: 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth RV. 2016. Least‐squares means: the R package lsmeans. Journal of Statistical Software 69: 1–33. [Google Scholar]
- Loreau M, de Mazancourt C. 2013. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecology Letters 16: 106–115. [DOI] [PubMed] [Google Scholar]
- Lu J, Robert CAM, Lou Y, Erb M. 2016. A conserved pattern in plant‐mediated interactions between herbivores. Ecology and Evolution 6: 1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Day M. 1998. Corn insect pests: a diagnostic guide. Columbia, MO, USA: University of Missouri‐Columbia. [Google Scholar]
- Ohgushi T. 2005. Indirect interaction webs: herbivore‐induced effects through trait change in plants. Annual Review of Ecology, Evolution, and Systematics 36: 81–105. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. 2016. vegan: Community Ecology Package. R package version 2.4‐1 [WWW document] URL https://CRAN.R-project.org/package=vegan. [accessed 7 September 2016].
- Poelman EH, Broekgaarden C, Van Loon JJA, Dicke M. 2008. Early season herbivore differentially affects plant defence responses to subsequently colonizing herbivores and their abundance in the field. Molecular Ecology 17: 3352–3365. [DOI] [PubMed] [Google Scholar]
- Poelman EH, Dicke M. 2014. Plant‐mediated interactions among insects within a community ecological perspective In: Voelckel C, Jander G, eds. Annual plant reviews insect plant interactions. New York, NY, USA: Wiley, 309–337. [Google Scholar]
- Robert CAM, Erb M, Duployer M, Zwahlen C, Doyen GR, Turlings TCJ. 2012a. Herbivore‐induced plant volatiles mediate host selection by a root herbivore. New Phytologist 194: 1061–1069. [DOI] [PubMed] [Google Scholar]
- Robert CAM, Erb M, Hibbard BE, Wade French B, Zwahlen C, Turlings TCJ. 2012b. A specialist root herbivore reduces plant resistance and uses an induced plant volatile to aggregate in a density‐dependent manner. Functional Ecology 26: 1429–1440. [Google Scholar]
- Robert CAM, Veyrat N, Glauser G, Marti G, Doyen GR, Villard N, Gaillard MDP, Kollner TG, Giron D, Body M et al 2012c. A specialist root herbivore exploits defensive metabolites to locate nutritious tissues. Ecology Letters 15: 55–64. [DOI] [PubMed] [Google Scholar]
- Schäfer M, Fischer C, Meldau S, Seebald E, Oelmüller R, Baldwin IT. 2011. Lipase activity in insect oral secretions mediates defense responses in Arabidopsis. Plant Physiology 156: 1520–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler R, Badenes‐Pérez FR, Broekgaarden C, Zheng S‐J, David A, Boland W, Dicke M. 2012. Plant‐mediated facilitation between a leaf‐feeding and a phloem‐feeding insect in a brassicaceous plant: from insect performance to gene transcription. Functional Ecology 26: 156–166. [Google Scholar]
- Stam JM, Kroes A, Li Y, Gols R, van Loon JJA, Poelman EH, Dicke M. 2014. Plant interactions with multiple insect herbivores: from community to genes. Annual Review of Plant Biology 65: 689–713. [DOI] [PubMed] [Google Scholar]
- Thaler JS, Fidantsef AL, Bostock RM. 2002. Antagonism between jasmonate‐ and salicylate‐mediated induced plant resistance: effects of concentration and timing of elicitation on defense‐related proteins, herbivore, and pathogen performance in tomato. Journal of Chemical Ecology 28: 1131–1159. [DOI] [PubMed] [Google Scholar]
- Utsumi S, Ando Y, Miki T. 2010. Linkages among trait‐mediated indirect effects: a new framework for the indirect interaction web. Population Ecology 52: 485–497. [Google Scholar]
- Viswanathan DV, Lifchits OA, Thaler JS. 2007. Consequences of sequential attack for resistance to herbivores when plants have specific induced responses. Oikos 116: 1389–1399. [Google Scholar]
- Viswanathan DV, Narwani AJT, Thaler JS. 2005. Specificity in induced plant responses shapes patterns of herbivore occurrence on Solanum dulcamara . Ecology 86: 886–896. [Google Scholar]
- Wang M, Biere A, van der Putten WH, Bezemer TM. 2014. Sequential effects of root and foliar herbivory on aboveground and belowground induced plant defense responses and insect performance. Oecologia 175: 187–198. [DOI] [PubMed] [Google Scholar]
- Winz RA, Baldwin IT. 2001. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. IV. Insect‐induced ethylene reduces jasmonate‐induced nicotine accumulation by regulating putrescine N‐Methyltransferase transcripts. Plant Physiology 125: 2189–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst S, van der Putten WH. 2007. Root herbivore identity matters in plant‐mediated interactions between root and shoot herbivores. Basic and Applied Ecology 8: 491–499. [Google Scholar]
- Xiao Y, Wang Q, Erb M, Turlings TCJ, Ge L, Hu L, Li J, Han X, Zhang T, Lu J et al 2012. Specific herbivore‐induced volatiles defend plants and determine insect community composition in the field. Ecology Letters 15: 1130–1139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Infestation by D. v. virgifera does not change aboveground damage by S. frugiperda larvae.


