Table 1.
ISES Evaluation of Bidentate Ligands for Ni(0)-Mediated Allylic Substitution.
| |||
|---|---|---|---|
| a no. | bidentate ligand | bΔO.D.340/time (mAbs/min) | c% conv |
| 1 | DPPB (6) | 132 ± 16 | 71 |
| 2 | (S,S)-SKEWPHOS (5b) | 64 ± 12 | 39 |
| 3 | (R)-BINAP (15a) | 45 ± 10 | 28 |
| 4 | (R,R)-Me-DUPHOS (10a) | 30 ± 1 | 22 |
| 5 | (S)-QUINAPd | 18 | f |
| 6 | (S,S)-Di-t-Bu-boxe | 15 ± 2 | f |
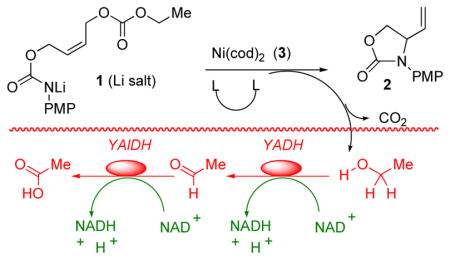
Conditions for the biphasic ISES screen (YADH = yeast alcohol dehydrogenase and YAlDH = yeast aldehyde dehydrogenase) as described in the Supporting Information.
Obs’d rates (10 min) of NADH formation in units of ΔO.D.340 min−1. Unless otherwise indicated, ISES slopes are reported as mean ± SD (duplicate runs).
Reaction conditions: 200 mM 1, 10 mol% Ni(cod)2, 20 mol% ligand, LiHMDS (1 eq.), THF, rt, 15 min. Product:educt ratio estimated by NMR following work-up.
Ligand is (S)-1-(2-diphenylphosphino-1-naphthyl)isoquinoline.
Ligand is 2,2′-methylenebis[(4S)-4-t-butyl-2-oxazoline].
Trace product (crude NMR).
