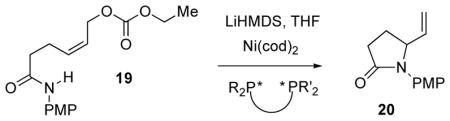
Table 3.
Allylic Aminations to Vigabatrin Derivative 20
| ||||
|---|---|---|---|---|
| a no. | ligand | yieldb | eec | confd |
| 1 | (R,R)-Me-DUPHOS (10a) | 93% | 66% | R |
| 2 | (S,S)-iPr-DUPHOS (10b) | 90% | 47% | R |
| 3 | (S,S)-Et-FerroTANE (11) | 28% | 8% | S |
| 4 | JOSIPHOS-type (12a) | 55% | 31% | R |
| 5 | JOSIPHOS-type (12b) | 28% | 74% | R |
| 6 | JOSIPHOS-type (12c) | 23% | 22% | S |
| 7 | JOSIPHOS-type (12d) | 73% | 5% | S |
| 8 | JOSIPHOS-type (12e) | nr | ||
| 9 | JOSIPHOS-type (12f) | 46% | 36% | R |
| 10 | WALPHOS-type (13a) | 8% | 32% | S |
| 11 | WALPHOS-type (13b) | 60% | 53% | S |
| 12 | (R)-BINAP (15a) | 70% | 43% | R |
| 13 | (R)-Tol-BINAP (15b) | 84% | 44% | R |
| 14e | (S)-MeO-BIPHEP (16a) | 97% | 53% | S |
| 15 | (R)-Me2-OMe-BIPHEP (16b) | 65% | 54% | R |
| 16 | (R)-tBu2-OMe-BIPHEP (16d) | 60% | 23% | R |
Reaction conditions: 67 mM 19, 10 mol% Ni(cod)2, 20 mol% ligand, LiHMDS (1 eq.), THF, rt, overnight.
Isolated yields of pure products. nr = no reaction observed.
ee’s determined by chiral HPLC (Chiralcel OD: hexane/i-PrOH 73/27).
Configuration assigned by correlation with the known γ-lactam, following PMP deprotection (CAN, MeCN, H2O): [α]23D (66% ee-chiral HPLC-see SI) –23.7 (EtOH, c 2.0); Lit.: [α]23D {(S)-isomer} +50.4 (EtOH, c 2.2) - ref. 6e.
No exogenous base used (i.e. no LiHMDS).
