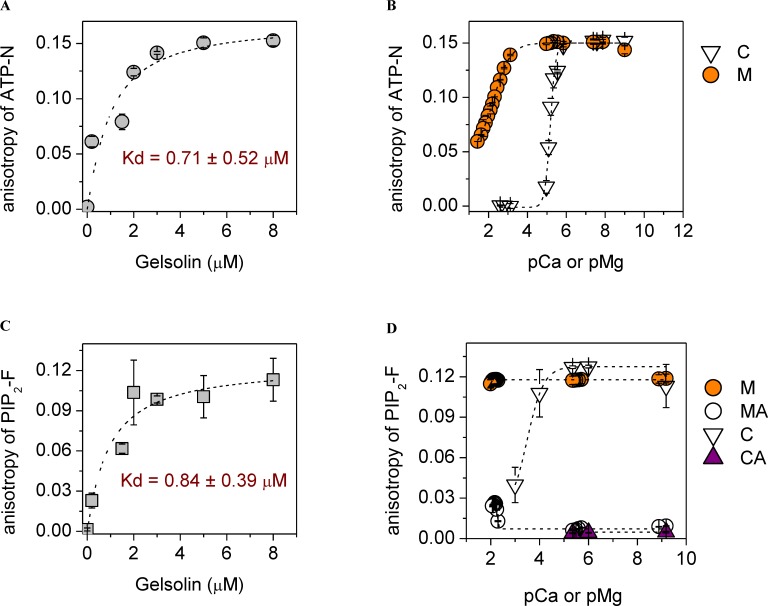
Fig 1. The interplay between calcium, magnesium, ATP and PIP2 for binding to gelsolin.
(a) In the absence of cations, the anisotropy of ATP-N (0.5 μM) increased and saturated by ~ 5 μM gelsolin. Dashed line shows the fit to the data (Eq 2). (b) Micromolar calcium levels (C, open triangles, inflection point at pCa 5.2) or millimolar magnesium levels (M, orange circles, inflection point at pMg 2.3) were able to reduce the anisotropy of ATP-N (0.5 μM) in the presence of gelsolin (5 μM), indicating the dissociation of ATP-N from gelsolin. Dashed line indicates sigmoidal fitting. (c) In the absence of cations, the anisotropy of PIP2-F (0.5 μM) increased and reached steady-state at ~ 5 μM gelsolin, indicating saturation of binding. Dashed line shows the fit to the data (Eq 2). (d) The anisotropy of PIP2-F (0.5 μM) in the presence of gelsolin (5 μM) was not changed on titration with magnesium (M, orange circles), but inclusion of 0.5 mM ATP (MA, white circles) lowered the anisotropy to the value characteristic to free PIP2-F, indicating complete dissociation of the gelsolin/PIP2-F complex by ATP. This effect was diminished by magnesium concentrations above 7 mM (pMg 2.15). In the absence of ATP, PIP2-F (0.5 μM) binds to gelsolin (5 μM), as revealed by the increase in anisotropy across a wide range of calcium concentrations (C, white triangles). Inclusion of ATP (0.2 mM, CA, purple triangles) lowered the anisotropy to the value characteristic to free PIP2-F, indicating complete dissociation of the gelsolin/PIP2-F complex by ATP.