Abstract
Study Design.
A retrospective review.
Objective.
To report the results of an alternative technique using a minimally invasive fusionless surgery. The originality is based on the progressive correction of the deformities with proximal and distal fixation and on the reliability of the pelvic fixation using iliosacral screws on osteoporotic bones.
Summary of Background Data.
Spinal deformities are common in neuromuscular diseases. Conventional treatment involves bracing, followed by spinal instrumented fusion. Growing rod techniques are increasingly advocated but have a high rate of complications.
Methods.
The technique relies on a bilateral double rod sliding construct anchored proximally by four hooks claws and distally to the pelvis by iliosacral screws through a minimally invasive approach. Hundred patients with neuromuscular scoliosis underwent the same fusionless surgery extended from T1 to the pelvis. The average age at initial surgery was 11 + 6 years. Diagnoses included cerebral palsy (61), spinal muscular atrophy (22), muscular dystrophy (10), and other neurological etiologies (7). Cobb angle and pelvic obliquity were measured before and after initial surgery, and at final follow-up. Complications were reviewed.
Results.
At latest follow-up 3 + 9 years (range 2 yr–6 + 3 yr), the mean Cobb angle improved from 89° to 35° which corresponds to 61% correction. Mean pelvic obliquity improved from 29° to 5°, which corresponds to 83% correction. Mean T1-S1 length increased from 30.02 to 37.28 cm. Mean preoperative hyper kyphosis was reduced from 68.44° to 33.29°. Complications occurred in 26 patients including mechanical complications (12) and wound infections (16). No arthrodesis was required at last follow-up.
Conclusion.
This original fusionless technique is safe and effective, preserving spinal and thoracic growth. It provides a significant correction of spinal deformities and pelvic obliquity with a reduced complications rate. The strength and stability of this modular construct over time allow the avoidance of final arthrodesis.
Level of Evidence: 4
Keywords: early onset scoliosis, fusionless surgery, iliosacral screws, minimally invasive technique, neuromuscular scoliosis, pelvic fixation, pelvic obliquity, progressive correction, telescopic rods, viscoelasticity
Neuromuscular spinal deformities are often progressive.1 Conservative treatment is not sufficient to maintain trunk and pelvic balance, and surgical treatment is frequently required. Early definitive spine fusion has the disadvantage of cessation of trunk growth with concomitant effects on lung development. Growth preserving spine surgeries are increasingly used but with high complication rates.
Minimally invasive surgery has been described in the treatment of adolescent idiopathic scoliosis.2 Such approaches are rarely reported in neuromuscular scoliosis patients.3 Our aim is to develop a minimally invasive approach for insertion of a strong and stable construct capable of further adjustments and avoidance of final fusion.
The purpose of this study is to present the results of our large consecutive series of 100 patients from 2011 to 2015. We report the degree of deformity correction and the incidence of surgical complications.
MATERIALS AND METHODS
Demographic Data
As described in Table 1, 100 consecutive patients with neuromuscular scoliosis underwent surgical correction through a minimally invasive surgical approach between 2011 and 2015, with a minimum of 2 years radiographic follow-up. Informed consent was obtained from parents/guardians of all participants. Procedures were conducted according to the ethics committee approval.
TABLE 1.
Descriptive Data of Patients Undergoing Minimally Invasive Surgery for Neuromuscular Scoliosis
| Mean age at surgery | 11 + 6 yr (range 5 + 1 yr to 21 + 3 yr) |
| Male/female | 58/42 |
| Mean operative time | 2 h 26 min (from 1 h 33 min to 3 h 39 min) |
| Mean operative time for rod lengthening | 44 min (from 26 min to 1 h 10 min) |
| Diagnosis | |
| Cerebral palsy | 61 |
| Spinal muscular atrophy | 22 |
| Muscular dystrophy | 10 |
| Other neurological etiologies | 7 |
| Other comorbidities | |
| Gastrostomy | 22 |
| Tracheostomy | 6 |
| Curve pattern | |
| Thoracolumbar left | 60 |
| Thoracolumbar right | 23 |
| Double major | 9 |
| Thoracic left | 4 |
| Thoracic right | 4 |
There were 58 men and 42 women.
The mean age at surgery was 11 + 6 years (range 5 + 1 to 21 + 3 yr).
Twenty-two patients had gastrostomy. Six patients had tracheostomy.
Diagnoses included cerebral palsy (61), spinal muscular atrophy (22), muscular dystrophy (10), and other neurological etiologies (7).
The curve patterns were left thoracolumbar in 60 cases, right thoracolumbar in 23 cases, double major in 9 cases, left thoracic in 4 cases, and right thoracic in 4 cases.
Study Design
It was a retrospective study using a consecutive patient cohort. We reviewed the preoperative and postoperative records of all patients and analyzed the correction of spinal deformity, pelvic obliquity, thoracic kyphosis, the T1-S1 length, and any perioperative or postoperative complications.
All patients were assessed every 6 months after surgery.
All data were obtained from medical records and radiographs.
Operative Procedures
All patients underwent general anesthesia and somatosensory and motor potentials monitoring.4
Intravenous antibiotics, cefazolin, and vancomycin were administrated perioperatively.
Skeletal traction was applied through skull tongs, with countertraction provided through boots. Asymmetric traction was used in cases of severe pelvic obliquity. Preoperative halo-gravity traction was also used for 4 weeks for stiff curves in 13 cases.
Two small midline skin incisions are made: the proximal incision centered on the upper thoracic spine and the distal incision centered on the lumbosacral junction.
The instrumentation used was a bilateral distraction construct EUROS (Figure 1):
Distal bilateral fixation is achieved using ilio-sacral screws which transfix a multiaxial connector.5 The length of the iliosacral screw is 45 to 75 mm, and the diameter is 7 mm. An approach is made through a transmuscular paramedian exposure to the posterior surface of the sacrum. The multiaxial connector is inserted in a trough created between the articular process of L5-S1 and the first posterior sacral foramen. A jig connected to the multiaxial connector is used to guide the insertion of an iliosacral screw through a small stab incision (Figure 2A–C).
Proximal bilateral fixation is achieved on the first thoracic vertebras, using a double claw consisting of supralaminar and pedicle hooks fixed on two adjacent vertebrae and separated by a single free vertebra. The sublaminar hook is first affixed to the hook pusher with the cap and inserted between the ligamentum flavum and the lamina without opening the spinal canal. The pedicle hooks are also affixed to the hook pusher, inserted manually under the articular facet, and then gently impacted with a hammer without any bone removal. The choice of this construct ensures the maximum efficiency against pullout forces, especially in cases with severe kyphosis.
A rod system composed of two long precurved rods (diameter 5.5 mm) inserted in an intramuscular way from the proximal wound to the distal one, and attached to the proximal hooks. Most of them were titanium alloy; however, cobalt chrome was used on heavy (>40 kg) or active patients. These rods are kept medial and attached through a side to side closed connector to an overlapping shorter lateral rod fixed to the distal multiaxial connector at S1. The amount of overlapping between the two rods corresponds to the required potential for lengthening of the construct. Cross-links are used proximally and distally to build a final stable frame construct.
Figure 1.
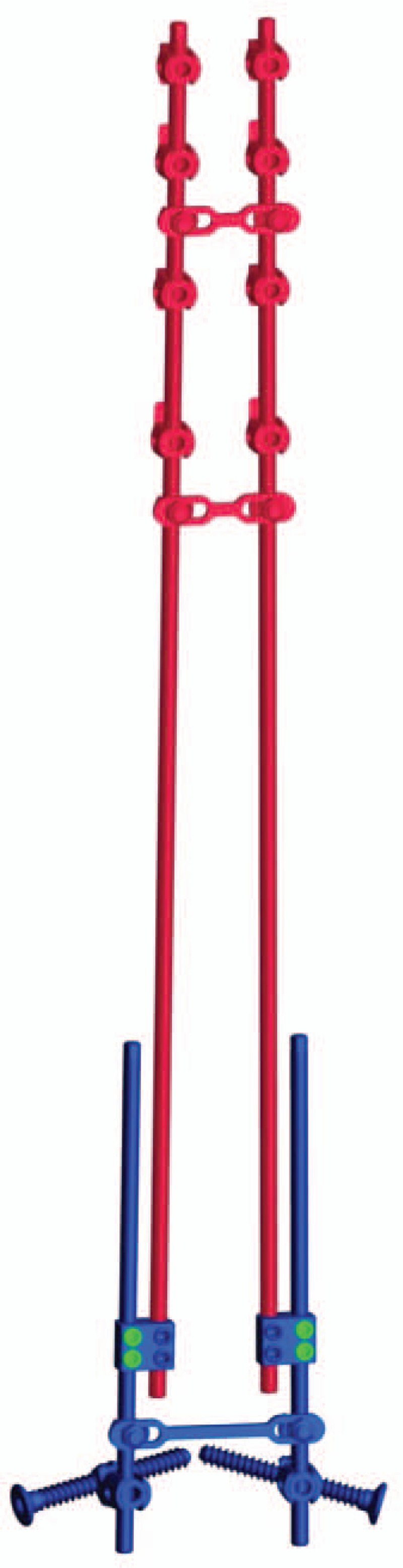
The bipolar construct anchored proximally by hooks in a double claw and distally by iliosacral screws through a minimally invasive approach.
Figure 2.
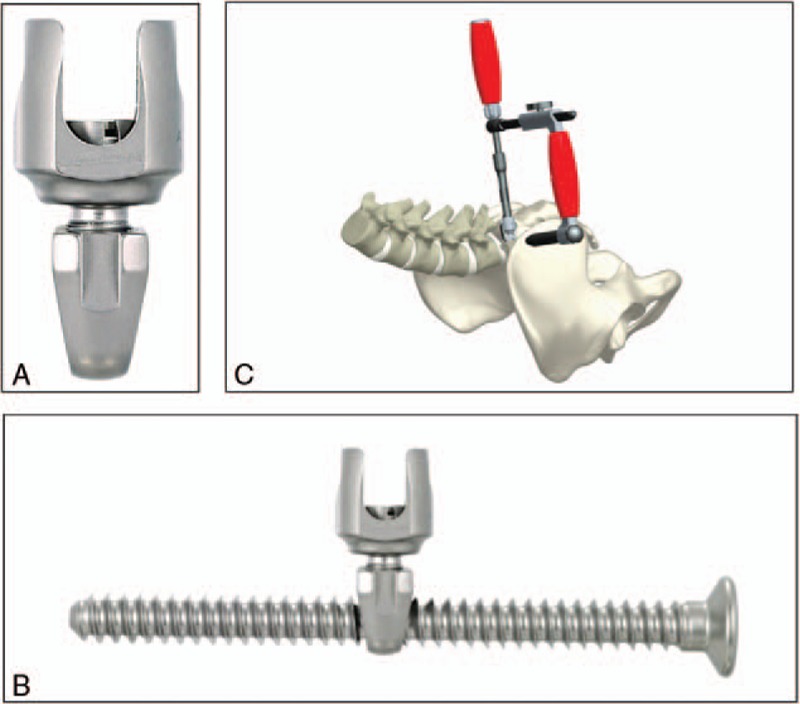
A, The multiaxial connector. B, The iliosacral screw. C, The jig used to guide the iliosacral screw placement.
Patients are allowed to sit without external support in the first postoperative days.
Rods are lengthened every 12 to 24 months using the previous distal incision to access the side-to-side connector. Rod lengthening of 15 to 30 mm is achieved at each session. Lengthening is performed to keep up with spinal growth or to achieve further correction of the deformity—especially in cases with severe pelvic obliquity.
Radiographic Evaluation
Standard sitting or standing posteroanterior and lateral radiograph were taken of the entire spine pre- and postoperatively, and at the follow-up visits.
The thoracolumbar curves, pelvic obliquity, and T1-S1 length were measured from the posteroanterior radiographs.
Pelvic obliquity was determined as described by Maloney et al6 (angle between a line drawn perpendicular from the middle of T1 to S1 and the iliac crest line).
T1-S1 length was determined as the horizontal distance in millimeters from the center of T1 to superior end plate of S1.
Thoracic kyphosis was measured from upper plate of T2 to lower endplate of T12 from the lateral radiographs.
Complications Evaluation
Complications that occurred intraoperatively, during hospital stay or within the 3 months after the operation were considered as perioperative complications.
Complications that occurred after 3 months postoperatively were considered as postoperative complications.
RESULTS
Clinical Findings
All patients were available for follow-up with an average of 3 + 9 year (range 2–6 + 3 yr).
The mean preoperative weight was 24.4 kg (range 14–41), and 29 kg (range 16–44) at latest follow-up.
Fifty-four patients underwent one rod lengthening; at a mean of 13 (6–27) months after initial surgery. Eighteen patients underwent two rod lenghtenings; with a mean of 16 (6–28) months after the first lengthening.
The average operative time for the initial surgery was 2 hours 26 minutes (from 1 h 33 min to 3 h 39 min).
The average operative time for rod lengthening was 44 minutes (from 26 min to 1 h 10 min).
After the index procedure 52 patients required intensive care unit (ICU), with a mean length of stay of 9 days (3–22 days). The mean duration of hospital stay on the orthopedic ward was 8.8 days (range 1–27).
After rod lengthening, 22 patients required ICU with a mean length of 4 days (from 1 to 9 days). The mean duration of hospital stay in the orthopedic ward was 4 days (1–9 days).
Perioperative tranexamic acid was used, and 24 patients received a blood transfusion.7–9
Radiographic Outcomes
All radiographic outcomes are summarized on Table 2.
TABLE 2.
Preoperative, Postoperative, and Final Follow-up Radiographic Outcomes
| Cobb angles | |
| Preoperative | 89° (25°–149°) |
| Postoperative | 33° (5°–65°) |
| Correction (%) | 63% |
| 1st rod lengthening | 33° (8°–61°) |
| 2nd rod lengthening | 35° (7° –71°) |
| Latest follow-up | 35° (6°–53°) |
| Correction (%) | 61% |
| Pelvic obliquity | |
| Preoperative | 29° (0°–80°) |
| Postoperative | 12° (−5°–35°) |
| Correction (%) | 41% |
| Latest follow-up | 5° (−5°–22 °) |
| Correction (%) | 83% |
| Hypercyphose T4-T12 >50° | |
| Preoperative | 69° (51°–102°) |
| Postoperative | 32° (13°–52°) |
| Latest follow-up | 33° (8.80°–50°) |
| Correction (%) | 51% |
| T1-S1 length | |
| Preoperative | 30,02 cm (22.30– 40.13 cm) |
| Postoperative | 35.98 cm (26.4–46.79 cm) |
| Latest follow-up | 37.28 cm (29.95–47.83 cm) |
The radiographic follow-up rate was 100% at latest follow-up.
The average preoperative Cobb angle measurement of the major curve was 89°(range, 25°–149°), and 33° postoperatively (range, 5°–65°), which corresponds to a 63% correction.
The mean pelvic obliquity before surgery was 29° (range 0°−80°) and 12° (range −5°–35°) postoperatively. It improved to a mean of 5° (range −5°–22°) at last follow-up, which corresponds to 83% of correction. Forty-seven patients have achieved a surgical goal of ±5° pelvic obliquity (Figure 3A–H and 4A–D).
Figure 3.
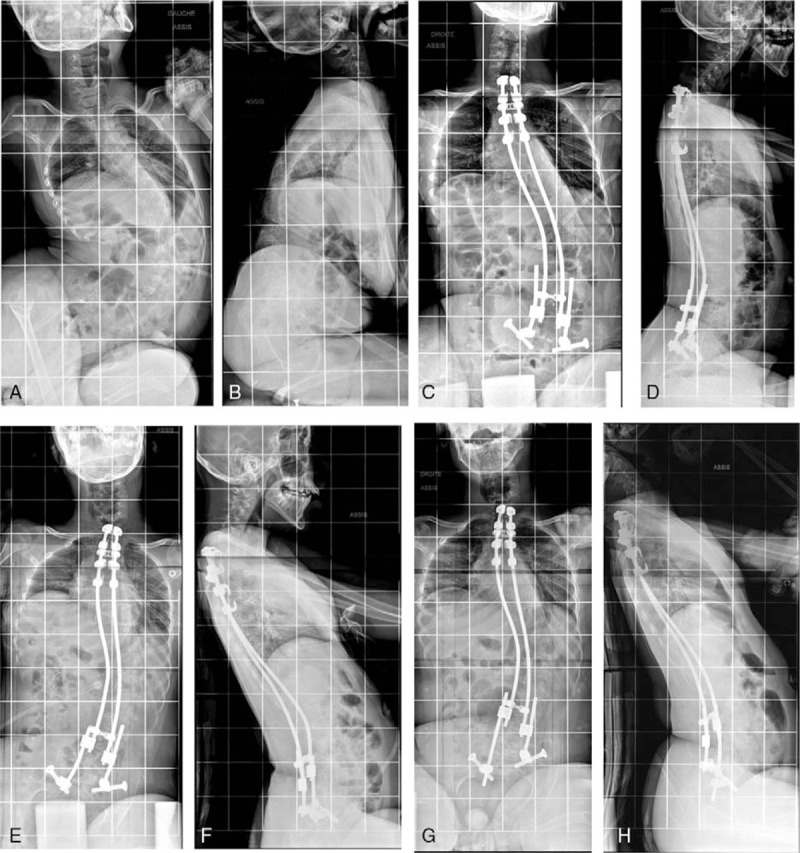
A–H, Radiographs of a boy with cerebral palsy. A/B, Preoperative x-rays with a 114° curve and 53° pelvic obliquity. C/D X-rays after initial surgery. E/F, X-rays after rod lengthening. G/H, X-rays after 3.5 years follow-up.
Figure 4.
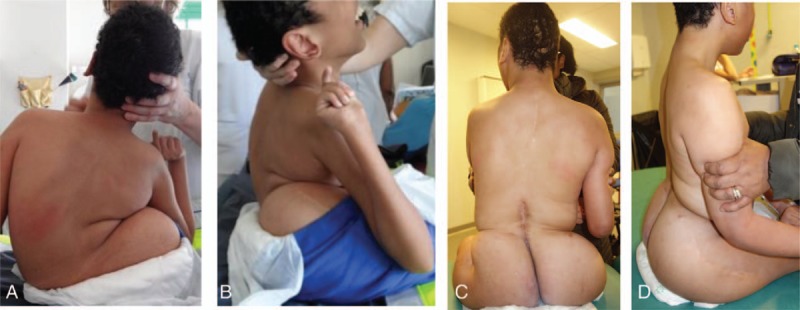
A–D, Pre- and postoperative photographs of this patient.
The mean postoperative curve was 33° (range, 8°–61°) after a first rod lengthening.
The mean postoperative curve was 35° (range, 7–71°) after a second rod lengthening.
The average Cobb angle at last follow-up was 35° (range 6°–53°) which corresponds to 61% correction. None of the patients had significant loss of correction during follow-up.
The mean T1-S1 length increased from 30.02 to 35.98 cm after initial surgery and 37.28 cm at latest follow-up.
Thirty-two patients were hyperkyphotic greater than 50° preoperatively. Mean preoperative hyper kyphosis was reduced from 69° to 33°. All patients had improved sagittal profile.
There were nine patients with radiological proximal kyphosis without any clinical consequences. They did not require any unplanned surgeries.
Complications
Twenty-six patients experienced complications (26%), which are summarized in Table 3.10–12
TABLE 3.
Complications of Minimally Invasive Surgery Classified According to the Modified Clavien-Dindo System
| Total | |
| Grade 1 | |
| Nerve's roots peroperative alerts | 12 |
| Grade 2 | |
| Blood transfusion | 24 |
| Grade 3 | |
| Wound infection | 16 |
| Implants dislodgments | 12 |
| Superior mesenteric artery syndrome | 2 |
| Pneumonia | 2 |
| Grade 4 | |
| Anaphylactic choc | 1 |
| Grade 5 | 0 |
Perioperative Complications
Anesthetic Complications
One patient underwent preoperative anaphylactic shock with good recovery.
Wound Infections
We reported 16 wound infections. All of them were diagnosed within the first 3 months of the operation.13–17
Two patients required implant removal for deep infection followed by a new implantation 1 year later with a good evolution.
Digestive Complication
Two patients had superior mesenteric artery syndrome, with complete resolution.
Pulmonary Complications
Two patients had pneumonia which was considered as major complications. They required noninvasive ventilation and antibiotic treatment in the ICU.
Neurological Compliactions
Twelve nerve's roots peroperative alerts on somatosensory potentials monitoring were observed. All improved after the immediate reduction of the correction. No clinical neurological complication occurred post operatively.
Postoperative Complications
Mechanical Complications
A total of 12 implant-related complications occurred.
They included four cases of prominence of the distal end of the rod, two cases of proximal hook dislodgment, and five cases related to the malposition of iliosacral screw.
There was one rod breakage. There were no cases of iliosacral screw dislodgement.
DISCUSSION
Unlike idiopathic scoliosis, neuromuscular spine deformity is related to a pathologic abnormality in muscle tone and muscle imbalance, which can cause a loss of sitting ability. Neuromuscular scoliosis often starts in the first years of life and early care-management of the deformity is essential.18
The common treatment plan consists of nonoperative options (bracing, physiotherapy) initially and spinal fusion surgery in adolescence.
Nevertheless, nonoperative management of patients with neuromuscular spinal deformity has no impact on muscle tone and cannot prevent curve progression—even for patients who are braced full time.19,20 The effect of this progressive spinal deformity on respiratory function leads to early respiratory insufficiency.21
At the onset of the prepubescent growth spurt, the progression of the deformity and the stiffness of the scoliosis become more difficult to treat. In these older patients, fusion surgery is longer, with more blood loss and with significant postoperative complications.
Rumalla et al,22 analyzed recent trends in the surgical management of neuromuscular scoliosis treated with spinal fusion. Between 2002 and 2011, a total of 2154 neuromuscular fusion cases were identified. The overall complication rate was 40.1%. They also found that the trends of increasing comorbidities, blood transfusions, and total costs in spinal fusion surgery for this population may warrant a more aggressive approach to these cases.
Our hypothesis was that spinal fusion may not be mandatory in nonambulatory neuromuscular patients where the mechanical stresses are reduced. The first Harrington instrumentation without fusion used for progressive scoliosis led to spontaneous fusion.23 Cahill et al24 reported that autofusion of the spine can occur in 89% children treated with growing rods. Recently, Jain et al25 reported that avoiding final surgery fusion at skeletal maturity was a viable option for patients treated with growing rods for early onset scoliosis who have satisfactory final alignment and trunk height.
Previously described fusionless scoliosis surgeries have also had high rates of complications (40%–73%).26,27 Because of neuromuscular patient's poor general health status (malnutrition or severe osteopenia), mechanical complications such as failure of the rib/pelvic anchors are common.
In Abol and Stuecker28 series, 20 children with neuromuscular scoliosis underwent percutaneous rib-to-pelvis Vertical Expandable Prosthetic Titanium Rib implantation. Nineteen patients suffered complications in the form of proximal cradle migration (5), implant breakage (5), deep wound infection (3), and dislodged iliac hooks (2). Sponseller et al29 evaluated the complications in 36 patients with neuromuscular scoliosis/syndromic scoliosis treated with growing rods anchored to the pelvis. Complications included deep wound infections (5), distal fixation complications (10), and rod breakages (6).
In our series, 12 cases of mechanical complications occurred, 5 of them during the first year related to the learning curve of the technique explained in Figure 5. The only case of rod breakage happened in a 17 years old dystonic boy, after 3 years of follow-up, probably due to the absence of distal side to side connectors in the construct. There were no cases of iliosacral screw dislodgment.
Figure 5.
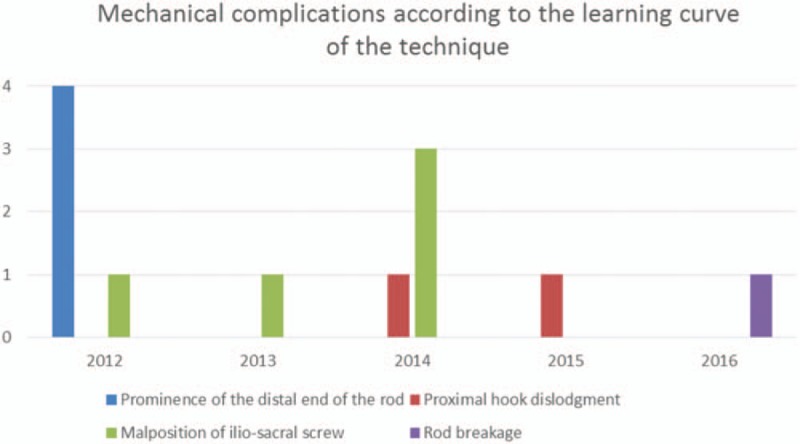
Learning curve of the technique.
The Cobb angle correction obtained in our series is comparable to the correction achieved in most other series using fusionless surgeries.30,31 However, we obtained a much better rate of pelvic obliquity correction (83%), thanks to the asymmetric rod lengthening provided by our expandable construct and the pelvic fixation with iliosacral screws. Consequently, the functional status of the patients is improved with a better balance in sitting position. In addition, most of our patients improved their general status, with a mean weight gain of 4.6 kg at latest follow-up as illustrated on Figure 6A–D.
Figure 6.
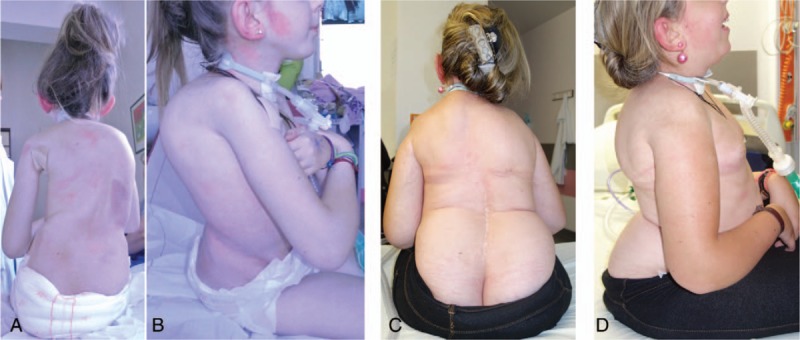
A–D, Pre- and postoperative photographs of patients surgically treated by the double rod distraction method.
Our original concept is based on an early surgery using a strong and stable expandable construct inserted with a minimally invasive approach.
This new technique is built on three key principles.
First, a biomechanical principle which relies on a strong and reliable proximal and distal fixation to correct the deformity under the effect of distraction between the two points of support. The power of the expandable construct allows a constant tension between the proximal and distal points of fixation. It allows symmetric or asymmetric distractions according to the residual spinal deformity or pelvic obliquity. The construct is especially stable thanks to an “Eiffel tower” shape structure with a divergent base, and a strong pelvic anchor using the iliosacral screws. The elasticity of the rods allows to more easily absorb the mechanical stresses along the length of the instrumentation. Moreover, the side to side connectors which join the rods have the possibility to rotate easily in case of torsional stresses, before the rods themselves. Thanks to this elasticity, we have noticed only one rod breakage in our series of 100 patients. The poly axial mobility of iliosacral connectors can also absorb the mechanical stresses at the distal end of the construct due to a higher lever arm effect on this long construct. The mechanical constraints on the rods or on the pelvis fixation can therefore be prevented. In contrast with our flexible construct, spinal fusion surgery requires rigid fixations which can lead to rod fracture due to stress concentrations in cases of pseudarthrosis.
Secondly, the biological principle is based on the minimally invasive approach. The preservation of the intermediate area between the proximal and distal fixation avoids early fibrosis and autofusion that could lead to cessation of growth and early stiffness of the spine. The preservation of this intermediate area also preserves spinal mobility in this segment, which allows for a better correction during the rod lengthening procedures.
Finally, the principle of spinal derotation through distraction is based on the viscoelastic relaxation of trunk's soft tissues, under the effect of the permanent distraction force maintained between the two extremities of the construct. This effect allows for a better correction and a reduction of perioperative neurologic complications compared to a one time aggressive spinal fusion.32
The correction of the spinal deformities through this method seems to follow two steps. During the first postoperative year, the instrumentation needs to be stable and stay in place until the bone remodeling process occurs around the implants and strengthens the points of anchor. During the next 5 to 6 years, the intermediate area maintains its mobility and allows a progressive correction of spinal and pelvic deformity in the frontal and sagittal planes, thanks to symmetric or asymmetric lengthening of the rods. Finally, the spine becomes stiff under the influence of the permanent presence of rods and remains in the corrected position. The final result is thus similar to a spinal fusion with respect to anatomic and functional aspects, but obtained after several years during which we have the possibility to improve the correction. Moreover, the correction of the pelvic obliquity is much better. In these conditions, we do not recommend removing the construct at maturity.
CONCLUSION
The results of this original fusionless minimally invasive technique are promising, providing a significant correction of spinal deformities and pelvic obliquity with a lower complication rate.
This technique has a great impact on treatment cost through shorter hospital stay—especially in ICUs and a lower cost of implants.
In addition, an earlier surgery makes the use of braces unnecessary and improves the patient's comfort and quality of life.
The stability of the construct over time and the avoidance of a final arthrodesis suggest that this technique could be an attractive alternative. A longer follow-up is required to confirm the results of this original technique.
Key Points
This fusionless double ends technique is safe and effective with a significant correction of Cobb angle and pelvic obliquity.
The global complications rate is reduced compared to arthrodesis.
The expandable modular construct provides symmetric or asymmetric distractions according to the residual spinal deformity or pelvic obliquity.
The viscoelastic relaxation of trunk's soft tissues allows a progressive correction and a reduction of per operative neurologic complications.
This construct is enough strong and stable to avoid final arthrodesis.
Footnotes
The device(s)/drug(s) is/are FDA-approved or approved by corresponding national agency for this indication.
No funds were received in support of this work.
Relevant financial activities outside the submitted work: royalties.
References
- 1.Thometz JG, Simon SR. Progression of scoliosis after skeletal maturity in institutionalized adults who have cerebral palsy. J Bone Joint Surg Am 1988; 70A:1290–1296. [PubMed] [Google Scholar]
- 2.Sarwahi V, Horn JJ, Kularni PM, et al. Minimally invasive surgery in patients with adolescent idiopathic scoliosis: is it better than standard approach? A two year follow-up study. Clin Spine Surg 2014; 29:331–340. [DOI] [PubMed] [Google Scholar]
- 3.Sarwahi V, Amaral T, Wendolowski S, et al. Minimally invasive scoliosis surgery: a novel technique in patients with neuromuscular scoliosis. Biomed Res Int 2015; 2015: 481945. doi: 10.1155/2015/481945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammet TC, Boreham B, Quraishi NA, et al. Intraoperative spinal cord monitoring during the surgical correction of scoliosis due to cerebral palsy and other nerumuscular disorders. Eur Spine J 2013; 22:S38–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miladi LT, Ghanem IB, Draoui MM, et al. Iliosacral screw fixation for pelvic obliquity in neuromuscular scoliosis. A long term follow up study. Spine (Phila Pa 1976) 1997; 22:1722–1729. [DOI] [PubMed] [Google Scholar]
- 6.Maloney WJ, Rinsky LA, Gamble JG. Simultaneous correction of pelvic obliquity, frontal plane, and sagittal plane deformities in neuromuscular scoliosis using a unit rod with segmental sublaminar wires: a preliminary report. J Pediatr Orthop 1990; 10:742–749. [DOI] [PubMed] [Google Scholar]
- 7.Edler A, Murray DJ, Forbes RB. Blood loss during posterior spinal fusion surgery in patients with neuromuscular disease: is there an increased risk? Paediatr Anaesth 2003; 13:818–822. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K, Lenke LG, Daubs MD, et al. Is spine deformity surgery in patient's with spastic cerebral palsy truly beneficial? A patient/parent evaluation. Spine (Phila Pa 1976) 2009; 34:2222–2232. [DOI] [PubMed] [Google Scholar]
- 9.Murphy NA, Firth S, Jorgenson T, et al. Spinal surgery in children with idiopathic and neuromuscular scoliosis. What's the difference? J Pediatr Orthop 2006; 26:216–220. [DOI] [PubMed] [Google Scholar]
- 10.Barsdord AI, Sproule DM, Kaufmann P. Scoliosis surgery in children with neuromuscular disease: findings from the US national inpatient sample, 1997–2003. Arch Neurol 2010; 67:231–235. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Wu C, Andersen T, et al. Prevalence of complications in neuromuscular scoliosis surgery: a literature meta-analysis from the past 15 years. Eur Spine J 2013; 22:1230–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reames DL, Smith JS, Fu KM, et al. Complications in the surgical treatment of 19,360 cases of pediatric scoliosos: a review of the Scoliosis Research Society Morbidity and Mortality database. Spine (Phila Pa 1976) 2011; 36:1484–1491. [DOI] [PubMed] [Google Scholar]
- 13.Benson ER, Thomson J, Smith BG, et al. Results of morbidity in a consecutive series of patients undergoing spinal fusion for neuromuscular scoliosis. Spine (Phila Pa 1976) 1998; 23:2308–2317. [DOI] [PubMed] [Google Scholar]
- 14.Szoke G, Lipton G, Miller F, et al. Wound infection after spinal fusion in children with cerebral palsy. J Pediatr Orthop 1998; 18:727–733. [PubMed] [Google Scholar]
- 15.Ramo BA, Roberts DW, Tuason D, et al. Surgical site infection after posterior spinal fusion for neuromuscular scoliosis: a thirty year experience at a single institution. J Bone Joint Surg Am 2014; 96:2038–2048. [DOI] [PubMed] [Google Scholar]
- 16.Master DL, Connie PK, Jochen SH, et al. Wound infections after surgey for neuromuscular scoliosis: risk factors and treatment outcomes. Spine (Phila Pa 1976) 2011; 36:E179–E185. [DOI] [PubMed] [Google Scholar]
- 17.Sponseller PD, LaPorte DM, Hungerford MW, et al. Deep wound infections after neuromuscular scoliosis surgery: a multicenter study of risk factors and treatment outcomes. Spine (Phila Pa 1976) 2000; 25:2461–2466. [DOI] [PubMed] [Google Scholar]
- 18.Saito N, Ebara S, Ohotsuka K, et al. Natural history of scoliosis in spastic cerebral palsy. Lancet 1998; 351:1687–1692. [DOI] [PubMed] [Google Scholar]
- 19.Miller A, Temple T, Miller F. Impact of orthoses on the rate of scoliosis progression in children with cerebral palsy. J Pediatr Orthop 1996; 16:332–335. [DOI] [PubMed] [Google Scholar]
- 20.Zimbler S, Craig CL, Harris S. Orthotic management of severe scoliosis in spastic neuromuscular disease: results of treatment. Orthop Trans 1982; 6:70. [Google Scholar]
- 21.Mayer OH. Scoliosis and the impact in neuromuscular disease. Paediatr Respir Rev 2015; 16:35–42. [DOI] [PubMed] [Google Scholar]
- 22.Rumalla K, Yarbrough CK, Pugey AJ. Spinal fusion for pediatric neuromuscular scoliosis: national trends, complications and in hospital outcomes. J Neurosurg Spine 2016; 25:500–508. [DOI] [PubMed] [Google Scholar]
- 23.Moe JH, Kharrat K, Winter RB. Harrington instrumentation without fusion plus external orthotic support for the treatment of difficult curvature problems in young children. Clin Orthop Rel Res 1984; 35–45. [PubMed] [Google Scholar]
- 24.Cahill PJ, Marvil S, Cuddihy L, et al. Autofusion in the immature spine treated with growing rods. Spine (Phila Pa 1976) 2010; 35:E1199–E1203. [DOI] [PubMed] [Google Scholar]
- 25.Jain A, Sponseller PD, Flynn JM. Avoidance of “final” surgical fusion after growing rod treatment for early onset scoliosis. J Bone Joint Surg Am 2016; 98:1073–1078. [DOI] [PubMed] [Google Scholar]
- 26.Bess S, Akbarnia BA, Thompson GH. Complications of growing rod treatment for early onset scoliosis: analysis of one hundred and forty patients. J Bone Joint Surg Am 2010; 92:2533–2543. [DOI] [PubMed] [Google Scholar]
- 27.Akbarnia BA, Marks DS, Boachie-Adjei OI. Dual growing rod technique for the treatment of progressive early onset scoliosis: a multicenter study. Spine (Phila Pa 1976) 2005; 30 (17 suppl):S46–S57. [DOI] [PubMed] [Google Scholar]
- 28.Abol O, Stuecker R. Bilateral rib to pelvis Eiffel tower VEPTR construct for children with neuromuscular scoliosis: a preliminary report. Spine J 2014; 14:1183–1191. [DOI] [PubMed] [Google Scholar]
- 29.Sponseller PD, Yang JS, Thompson GH. Pelvic fixation of growing rods: comparison of constructs. Spine (Phila Pa 1976) 2009; 34:1706–1710. [DOI] [PubMed] [Google Scholar]
- 30.Tsirikos AI, Lipton G, Chang WN, et al. Surgical correction of scoliosis in pediatric patients with cerebral palsy using the unit rod instrumentation. Spine (Phila Pa 1976) 2008; 33:1133–1140. [DOI] [PubMed] [Google Scholar]
- 31.Tsirikos AI, Mains E. “surgical correction of spinal deformity in patients why cerebral palsy using pedicule screws instrumentation”. J Spinal Disord Tech 2012; 25:404–408. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton DK, Smith JS, Sansur CA, et al. Rate of new neurological deficit associated with spine surgery based on 108,419 procedures: a report of the scoliosis research society morbidity and mortality committee. Spine (Phila Pa 1976) 2011; 36:1218–1228. [DOI] [PubMed] [Google Scholar]


