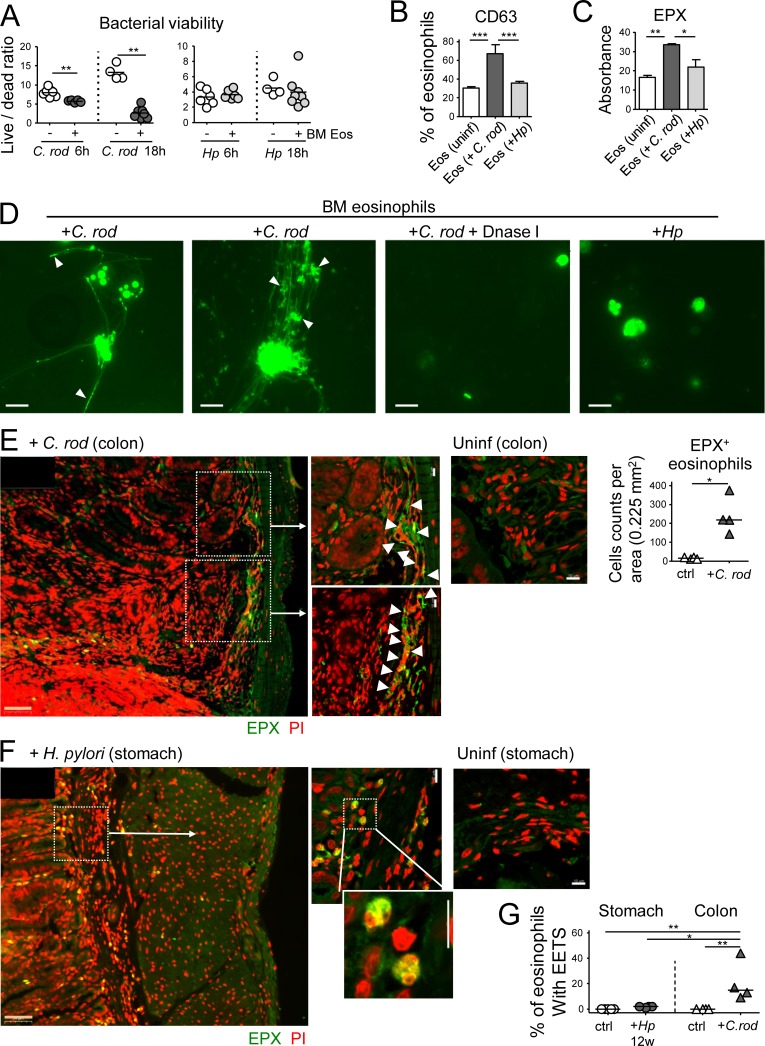
Figure 7.
Eosinophils contribute to C. rodentium, but not H. pylori control through degranulation and extracellular trap formation. (A) In vitro differentiated eosinophils were co-cultured with either C. rodentium or H. pylori for 6 or 18 h; bacterial viability was determined by live/dead staining and FACS. (B) CD63 expression as a marker of eosinophil degranulation, as assessed by FACS of in vitro infected and naive eosinophil cultures. (C) EPX expression, as assessed by ELISA of the supernatants of eosinophil cultures. (D) Eosinophil extracellular trap (EET) formation by eosinophils infected with C. rodentium, but not H. pylori; white arrowheads point to bacteria caught in EETs. DNase I treatment dissolves EETs. (E and F) Representative confocal immunofluorescence microscopy images of FFPE sections of a C. rodentium–infected colon (12 d, E) and an H. pylori–infected stomach (12 wk, F), along with uninfected control sections of both tissues (EPX, green; propidium iodide, PI, red). Arrows point to regions of high eosinophil density and of colocalized extracellular EPX and EETs. The quantification of eosinophils in C. rodentium–infected colons is shown in E. Bars, 50 µm in low magnification (orange), 10 µm in high magnification images (white). (G) Quantification of eosinophils with EETs in stomachs and colons of four H. pylori–infected and four C. rodentium–infected mice relative to their uninfected controls. Statistics were calculated by Mann-Whitney test in A–E and by one-way ANOVA with Bonferroni’s correction in G. *, P < 0.05; **, P < 0.01; ***, P < 0.001.