Figure 1.
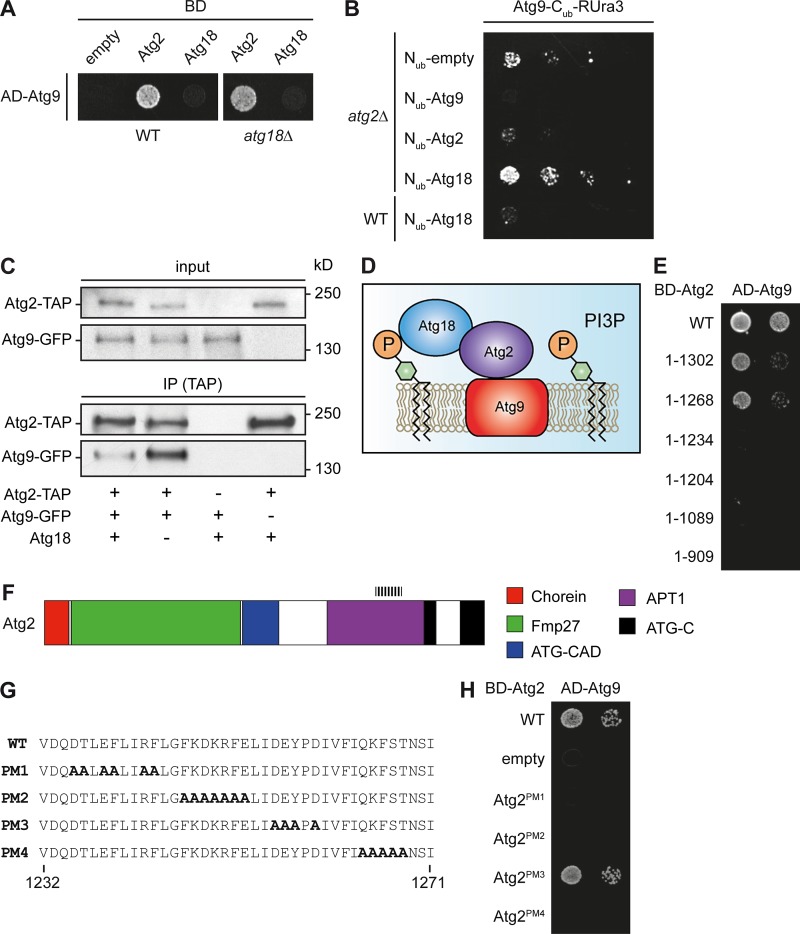
Atg2 and Atg9 directly interact. (A) Atg2–Atg9 interaction in different Y2H strains. Plasmids carrying the ATG2 or ATG9 gene fused with the BD or AD domains of the transcription factor Gal4, respectively, were transformed into Y2H WT (PJ69-4A) or atg18Δ (FRY382) strains. The pGBDU-C1 plasmid (empty) was used as a negative control. (B) Recapitulation of the Atg2–Atg9 interaction using the split-ubiquitin assay. All the split-ubiquitin constructs—pATG9_Cub_RURA3_Met313, pATG9_Nub_CUP_314, pATG18_Nub_CUP_314, and pATG2_Nub_Cub_314—were cotransformed into either WT (SEY6210) or atg2Δ (FRY383) cells. The pNub_CUP_314 plasmid was used as a negative control. (C) Atg18 is not required for the Atg2–Atg9 interaction. Cell extracts from atg2Δ atg9Δ (yDP29), atg2Δ Atg9-GFP (yDP191), and atg2Δ atg18Δ Atg9-GFP (yDP264) strains transformed with an empty vector (pRS315) or a plasmid expressing TAP-tagged Atg2 were subjected to pulldown experiments as described in Materials and methods. Immunoisolates were analyzed by Western blotting using anti-GFP and anti-TAP antibodies. (D) Model of the Atg9–Atg2–Atg18 complex. (E) A stretch of 34 amino acids between positions 1,232 and 1,268 of Atg2 is essential for the interaction with Atg9. Plasmids expressing the Atg21–1,302, Atg21–1,268, Atg21–1,204, Atg21–1,089, and Atg21–909 truncations were cotransformed with the vector carrying AD-Atg9 into the WT strain (PJ69-4A) before being assayed on the test plates. (F) Structural organization of Atg2 in domains as proposed (Kaminska et al., 2016). Through homology search (Finn et al., 2016), it appears that Atg2 possesses a Chorein-N domain (PF12624), a region with similarity to the mitochondrial protein FMP27 predicted to form a solenoid structure, an ATG2-CAD domain (PF13329) with unknown function, and a part similar to the Golgi APT1 protein of maize (PF10351). Additionally, the C terminus of Atg2 contains a region of high homology with the two mammalian Atg2 orthologues. It is composed of two ATG-C domains (PF09333) of unknown function. The first domain is truncated and lacks the distal part, whereas the second one is intact. The dashed lines indicate the identified region of Atg2 where the amino acids essential for its binding to Atg9 are localized. (G) Point mutants in Atg2. The Atg2 amino acid sequence between residues 1,232 and 1,271 is shown. The four Atg2 point mutants (PM1, PM2, PM3, and PM4) generated by replacing the charged and polar amino acids with alanines are indicated. The introduced alanines are in bold. (H) Interaction of point mutants with Atg9. BD-tagged Atg2 point mutants Atg2PM1, Atg2PM2, Atg2PM3, and Atg2PM4 were tested for their ability to bind AD-Atg9 in the WT strain (PJ69-4A) by Y2H assay. Only Atg2PM3 was able to interact with Atg9.