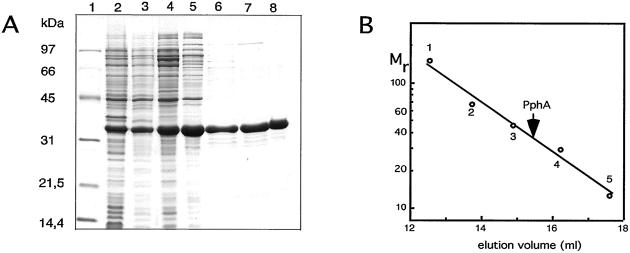
Figure 4.
(A) Purification of overproduced PphA shown by Coomassie-blue-stained 12.5% SDS/PAGE. The lanes correspond to the different purification steps as described in Materials and Methods. 1, molecular weight marker; 2, S10; 3, S100; 4, first (NH4)2SO4 precipitate; 5, DEAE Sepharose Fast Flow-pool; 6, UnoQ-pool; 7, Methyl HIC-pool; 8, Superdex 200-pool. (B) Determination of the native molecular size of PphA by gel-permeation chromatography. PphA and proteins used as size standards (1, alcohol dehydrogenase, 150 kDa; 2, BSA, 67 kDa; 3, ovalbumin, 43 kDa; 4, carbonic anhydrase, 29 kDa; 5, cytochrome C, 12.4 kDa) were passed separately through a Superdex S200 h 10/30 column (Amersham Pharmacia) equilibrated in 20 mM Tris⋅Cl (pH 7.4), 150 mM NaCl, 5 mM MgCl2, 0.5 mM EDTA, 2 mM DTT, 1 mM benzamidine, and 1 mM ɛ-aminocaproic acid.