Abstract
Congenital heart defects (CHDs) are the most common birth defects (BDs) and account for nearly one-third of all BDs. The aim of this article was to investigate the epidemiology and major subtypes of CHDs in Hunan Province, China in the last 5 years.
CHD surveillance data from 2012 to 2016 were collected from 52 registered hospitals in Hunan. The prevalence rates of CHDs, incidence rates of CHDs combined with other BDs, and rates of termination of pregnancy (TOP) for CHDs among different regions, infant sexes, and maternal ages were calculated for both early fetuses (<28 weeks of gestation) and perinatal infants (PIs) (between 28 weeks of gestation and 7 days after birth). Both the constituent ratio and prevalence rates were computed among subtypes.
CHDs were found in 6289 out of 673,060 births. The overall prevalence was 93.44 per 10,000 PIs, with 19.27 and 74.17 per 10,000 in early fetuses and PIs, respectively. The risks of CHDs were higher in infants from urban areas than those from rural areas during the whole gestation and were higher in male infants than in female infants during the perinatal period. The total prevalence of CHDs increased significantly with maternal age (χ2 trend = 141.84, P < .05). Among fetuses in early gestation, there were 288 cases (22.21%) of CHDs combined with other BDs and 1292 cases (99.61%) of TOP for CHD. The 3 major subtypes of CHDs were ventricular septal defect (VSD) (22.06%), Tetralogy of Fallot (TOF) (9.43%), and atrioventricular septal defect (AVSD) (6.69%). Among PIs, there were 1541 cases (30.87%) of CHD diagnosed before delivery and 1184 cases (76.83%) were TOP. The 3 major subtypes were atrial septal defect (ASD) (42.81%), patent ductus arteriosus (PDA) (16.07%), and VSDs (15.21%).
The total prevalence of CHD in Hunan Province and the rate of TOP for CHD was high, especially for early-gestation fetuses. Pregnancies in urban women, male PIs, and maternal age were the risk factors for CHDs. Among early-gestation fetuses, the most common types were VSD, TOF, and ASD, and among PIs, the most common types were ASD, PDA, and VSD.
Keywords: congenital heart defects, epidemiology, subtypes, surveillance
1. Introduction
Congenital heart defects (CHDs) are the most common type of birth defect (BDs) and the leading cause of infant mortality. The incidence rates of CHDs reported in different studies vary from approximately 4 to 50 per 1000 live births.[1] Some subtypes of CHDs are mild diseases with relatively little need for medical care, but other subtypes are complicated diseases requiring great expertise in this field. Thus, understanding the epidemiology and major subtypes of CHDs is important for recommending valuable changes in health policies.[2]
The estimates of early CHD prevalence in China based on postnatal case assessment alone range from 2.7 to 6.6 per 1000 live births, lower than those reported in some developed countries.[3] Recently, hospital-based studies of prenatal CHD detection have been conducted in China.[4] The Ministry of Health of China initiated a national hospital-based Birth Defect Surveillance System in 1986 in order to track perinatal infants (PIs), including live births up to 7 days, fetal deaths, stillbirths or terminations of pregnancy due to fetal anomaly. CHDs were recorded in detail in this surveillance system. In Hunan, the surveillance system that involved 52 hospitals began in 1991 (selected by urban, rural and were uniformly distributed in 14 cities of Hunan province), and recorded information such as incidence, distribution, and determinants of BDs, including CHDs.[5]
Hunan has been confronted with tremendous challenges in BDs prevention in recent years. The prevalence rates of BDs were ranked third in China in 2011, fourth in 2012, and fifth in 2013.[6] In particular, almost one-third of BDs were CHDs.[7] Moreover, the patterns and incidence rates of CHDs differ geographically due to racial and ethnic factors.[8] However, studies of CHDs usually focus only on the late gestational period (>20 or 28 weeks) or live births.[8,9] The total prevalence of CHDs among all fetuses may be higher than that reported among live births alone or among fetuses later in gestation alone, since complex CHDs are common throughout the gestational period[10] and may result in spontaneous abortion or stillbirth, especially early in gestation. This study aimed at investigating the epidemiology and major subtypes of CHDs among all infants, including both early in gestation and during the perinatal period by using results from the surveillance system in Hunan for the last 5 years.
2. Methods
2.1. Study population and ethics statement
This study was cross-sectional study, involving all infants (including stillbirths, dead fetuses, or live births) during the perinatal period and CHD cases at <28 weeks of gestation born in the 52 registered hospitals of Hunan between 2012 and 2016. The CHD records were reported both on paper and online, and anonymized and de-identified prior to analysis. The records of CHD included the maternal and fetal characteristics, such as maternal census data, maternal age, fetus gestation, fetus sex, the subtype of CHD, outcome, and so on. This study was approved by the Medical Ethics Committee of Hunan Maternal and Child Health Hospital. Written informed consent was obtained from all participants’ guardians.
2.2. Criteria for CHD diagnosis
The diagnosis of CHDs was based on the Chinese National Criteria of Birth Defects and Tiny Deformities and confirmed within 7 days of birth (postural defects were excluded from the monitoring system). The main diagnostic tool for CHDs is echocardiography. The clinical diagnosis of CHD was finished within 7 days after delivery. Isolated patent foramen ovale (PFO) and PDA that failed to close during the supervised period were included. Experts from each registered hospital provided diagnostic technical support and were responsible for confirming the diagnoses. Complex CHDs were diagnosed in a higher-level hospital or with expert consultation. CHDs were coded according to the International Classification of Diseases Clinical Modification Codes, tenth edition (ICD-10).
2.3. Quality control
According to the require of Health and Family Planning Commission of China, the maternal and children's hospitals and health administrative departments audited the CHD cases and perinatal data. The monitoring hospitals were inspected and examined at quarterly intervals by county-level administrators and at half-yearly intervals by city-level or province-level administrators to ensure quality control and reduce the probability of misreporting or failure of reporting or missing data of reports. After quality control, we deleted some cases missing important information which we could not completed before analysis.
2.4. Statistical analysis
The prevalence rates of CHDs, incidence rates of CHDs combined with other BDs, and rates of TOP for CHD with 95% confidence intervals (CI) among the different regions, infant sexes, and maternal ages were calculated separately. Gestation was divided into early gestation (<28 weeks of gestation) and the perinatal period (between 28 weeks of gestation and 7 days after birth). The relationship of each maternal characteristic with CHDs was explored using the Chi-squared test and the crude odds ratio (OR). The proportion and prevalence rates of the major CHD subtypes were also presented and ranked. Prevalence estimates were reported as “per 10,000 PIs” (perinatal infants). The data were analyzed using SPSS 21.0 (SPSS, Chicago, IL) with the significance level at P < .05.
3. Results
3.1. Prevalence of CHDs in Hunan
A total of 673,060 PIs (approximately16.71% of the total births in Hunan Province, 673,060/4,027,317) were reported to the Birth Defect Surveillance System of Hunan, and of these, 1297 cases (20.62%) during early gestation and 4992 cases (79.38%) during the perinatal period were diagnosed as CHDs. The total prevalence rate of CHDs was 93.44 (95% CI: 91.38–95.50) per 10,000 PIs, while the average prevalence rates in early gestation and in the perinatal period were 19.27 (95% CI: 18.18–20.36) and 74.17 (95% CI: 72.07–76.27) per 10,000 PIs, respectively.
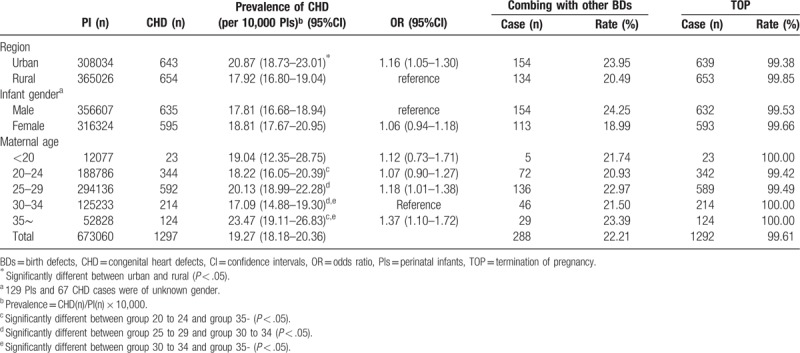
Among the fetuses early in gestation, the average prevalence rate of CHDs was significantly higher in urban areas versus rural areas (20.87 vs 17.92 per 10,000 PIs) (OR = 1.16, 95% CI: 1.05–1.30, P = .006). No significant difference was found between male and female infants (OR = 1.06, 95%CI: 0.94–1.18, P = .336). The prevalence rate of CHDs was lowest in the maternal age group of 30 to 34 and highest in the age group ≥35 (17.09 vs 23.47 per 10,000 PIs) (OR = 1.37, 95%CI: 1.10–1.72, P = .005) (Table 1).
Table 1.
Prevalence for CHDs and rate of TOP less than 28 weeks of gestation with maternal characteristics.
Among PIs, the average prevalence rate of CHDs was significantly higher in urban versus rural areas (104.24 vs 48.79 per 10,000 PIs) (OR = 2.15, 95%CI: 2.03–2.28, P < .01), in male versus female PIs (76.53 vs 71.32 per 10,000 PIs) (OR = 1.07, 95%CI: 1.02–1.14, P < .001) and for maternal age ≥30 vs <30 (OR = 2.07, 95%CI: 1.57–2.73, P < .001) (Table 2).
Table 2.
Prevalence of CHD and rate of TOP between 28 weeks of gestation and 7 days after birth according to different characteristics.
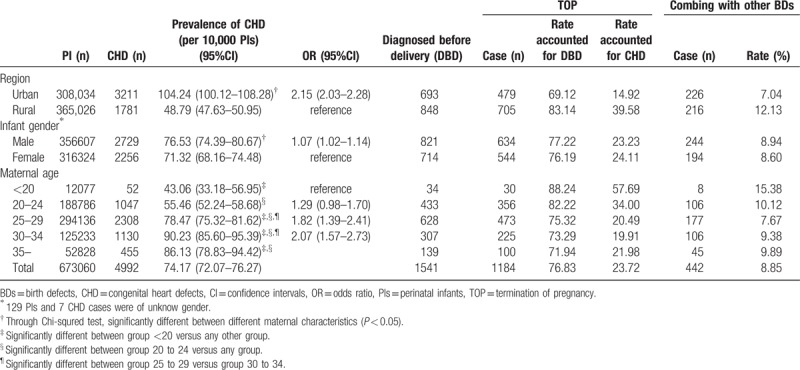
Combining the data for fetuses in early gestation with PIs, the total prevalence rate of CHDs significantly increased with maternal age (χ2 trend = 141.84, P < .001). Age group <20 as a reference, the ORs were 1.18 (group 20–24, 95% CI: 0.94–1.50), 1.59 (group 25–29, 1.27–2.01), 1.74 (group 30–34, 1.38–2.19), and 1.77 (group ≥35, 1.39–2.26).
3.2. Incidence rate of CHDs combined with other BDs and the rate of TOP for CHDs
Among the fetuses early in gestation, there were 288 cases (22.21%) of CHDs combined with other BDs and 1292 cases (99.61%) of TOP. Five cases (0.39%) were premature infants who were diagnosed with CHDs after delivery.
Among the PIs, there were 1541 cases (30.87%) of CHD diagnosed before delivery (DBD) and 1184 cases (76.83% of DBD cases, 23.72% of total CHD cases) were TOP for CHD. Among the CHD cases, 442 cases (8.85%) were combined with other BDs.
3.3. Major subtypes of CHDs in Hunan
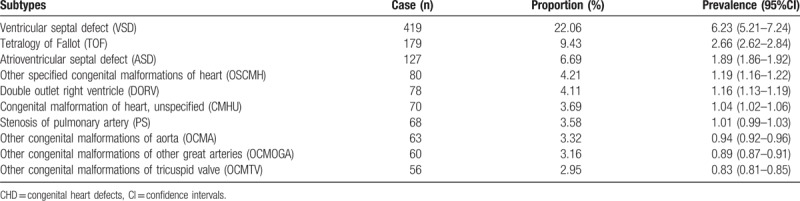
Among the fetuses early in gestation, the five major subtypes of CHDs were VSD (22.06%), TOF (9.43%), AVSD (6.69%), other specified congenital malformations of the heart (OSCMH) (4.21%), and double outlet right ventricle (DORV) (4.11%) (Table 3).
Table 3.
The major 10 subtypes of CHDs among fetuses less than 28 weeks of gestation.
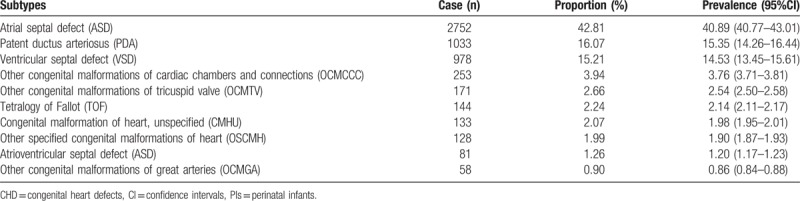
Among PIs, the 5 major subtypes of CHDs were ASD (42.81%), PDA (16.07%), VSD (15.21%), other congenital malformations of cardiac chambers and connections (OCMCCC) (3.94%), and other congenital malformations of the tricuspid valve (OCMTV) (2.66%) (Table 4).
Table 4.
The major 10 subtypes of CHDs among PIs.
4. Discussion
The total prevalence rate of CHDs (93.44 per 10,000 PIs, 95%CI: 91.38–95.50) observed in this study is higher than in most other studies.[11–13] For example, the overall prevalence of CHDs was 62.10 per 10,000 PIs in China in 2016[13] and 6.5 per 1000 births in Europe.[14] These differences may due to genetic, environmental, or socioeconomic factors or ethnic origin, and thus, they require further investigation.[15] In addition, unlike most other studies that only focused on PIs, the CHD cases in our study also include fetuses in early gestation. Furthermore, Hunan strictly complied with the Chinese National Criteria of Birth Defects and Tiny Deformities, whereas in some provinces, underestimates of the prevalence of some tiny deformities results in lower reported BD rates.
The prevalence of CHDs in urban Hunan was significantly higher than that in rural areas for both early gestation fetuses and PIs, similar to the state-level results that indicated a higher prevalence in urban versus rural areas in 2007.[16] This phenomenon can primarily be attributed to stronger overall health awareness and the wider use of prenatal diagnostic techniques in urban areas, which increase health care accessibility and reporting practices. Second, factors that increase the level of environmental exposure, such as industrialization and urbanization, affect the occurrence of CHDs in urban areas.[10] Similar to some previous studies,[13,17,18] we also found that the prevalence rate of CHDs was significantly higher in male versus female PIs. A study in Tuscany showed that male sex is an independent predictor of severe CHDs,[19] which might be explained by the interaction of sex hormones and system development.[20] However, there was no significant difference between male and female fetuses early in gestation. Perhaps because the effect of sex on CHDs is only detectable later in gestation, and this phenomenon deserves further investigation. Some previous studies have reported the impact of maternal age on the occurrence of CHDs. We found that the total prevalence of CHDs increased with maternal age. As reported, maternal perinatal diseases, maternal medication use (antibiotic use), advanced maternal age, low socioeconomic status, and paternal alcohol intake were significantly associated with the incidence of CHDs, with ORs ranging from 1.60 to 3.96.[21]
Among fetuses early in gestation, the 3 major subtypes of CHDs were VSD (22.06%), TOF (9.43%), and AVSD (6.69%), while the 3 major subtypes among PIs were ASD (42.81%), PDA (16.07%), and VSD (15.21%). One possible reason for this difference is that some defects were diagnosed after delivery, including PFO (classified as an ASD) and PDA. Meanwhile, some severe defects, such as TOF, were more accessible diagnosed before 28 weeks of gestation with better and more accessible echocardiography. Many surveillance studies indicate that the major subtype (approximately 30%–40%) of CHDs was VSD.[22,23] This finding may be influenced by the increasing use of echocardiography, which has led to a considerable increase in the detection rate of small VSD.
In our study, the rate of CHDs combined with other BDs was 22.21% during early gestation and 8.85% during the perinatal period. These data differ from the rate of 14.04% reported during the perinatal period in Bao’an, China.[24] In the research of Jansen et al,[25] the prevalence of isolated left-sided CHDs, combining copy number variants, is 33%. Because autopsies are seldom conducted in China and few chromosomal tests were performed in our study (0.57%, 36/6289), some other BDs, especially those associated with chromosomal disorders, were rarely found in our study.[26]
The rate of TOP due to CHDs varies considerably in different countries. CHDs are common and bring a burden of ongoing medical management for those affected. CHDs have led to the development of prenatal diagnosis policies. It is the parents’ option to choose whether to have an infant with CHD. In our study, the rate of TOP with CHD was 99.61% before 28 weeks of gestation, while it was 76.83% among DBD cases. In agreement with our data, a previous study reported 4366 cases that were diagnosed with the 11 most severe malformations (2 types were CHD). Of these, 64% (2806/4366) were diagnosed prenatally and 66% (1863/2806) resulted in TOP.[26] In some countries, such as South Africa[27] and Malta,[28] the proportion of TOP due to CHDs is small and TOP is even prohibited. These countries are completely opposed to TOP due to personal experiences or moral, ethical, or religious convictions. In these countries, TOP is regarded as controversial and remains a taboo topic, even though this procedure is becoming more acceptable and demanded.[27] In addition, a high prenatal detection rate and early prenatal diagnosis will increase the proportion of TOP.
This study comprehensively illustrates the epidemiological characteristics of CHDs in Hunan, the most populated province in China, and includes all cases of CHDs in the last 5 years throughout early gestation and the perinatal period. Thus, the prevalence of CHD observed in this study is closer to the reality. However, there are some limitations. First, this surveillance system does not include the TOP cases without BDs before 28 weeks of gestation, so the denominator in the prevalence of CHDs for early gestation is “PIs” rather than “all fetuses.” Second, for cultural reasons, autopsies are seldom conducted in China,[10] and chromosome tests were rarely used in our study, so some infants with CHDs combined with other BDs were failed to be identified. Thus, the observed rate of CHDs combined with other BDs may be underestimated.
Acknowledgments
The authors gratefully acknowledge all the members involved in the work of Hunan Birth Defect Surveillance. They also thank Li Dai for his kind help.
Author contributions
Conceptualization: Donghua Xie, Junqun Fang, Zhiyu Liu, Hua Wang.
Data curation: Donghua Xie, Zhenqiu Sun, Aihua Wang, LiliXiong.
Formal analysis: Zhenqiu Sun,Tubao Yang
Funding acquisition: Donghua Xie.
Methodology: Zhenqiu Sun,Tubao Yang
Resources: Zhiyu Liu, Junqun Fang, Hua Wang.
Supervision: Junqun Fang, Zhiyu Liu, Hua Wang.
Writing – original draft: Donghua Xie.
Writing – review and editing: Tubao Yang.
Footnotes
Abbreviations: ASD = atrial septal defect, AVSD = atrioventricular septal defect, BDs = birth defects, CHDs = congenital heart defects, CI = confidence intervals, DBD = diagnosed before delivery, DORV = double outlet right ventricle, OCMCCC = other congenital malformations of cardiac chambers and connections, OCMTV = other congenital malformations of the tricuspid valve, OR = odds ratio, OSCMH = other specified congenital malformations of the heart, PDA = patent ductus arteriosu, PFO = isolated patent foramen ovale, PIs = perinatal infants, TOF = Tetralogy of Fallot, TOP = termination of pregnancy, VSD = ventricular septal defect.
DX and JF have contributed equally to the work as the co-first authors.
ZL and HW have contributed equally to the work as the co-corresponding authors.
This work was supported by the Foundation of health and family planning commission of Hunan Province (B20180175).
The authors of this work have no conflicts of interest to disclose.
References
- [1].Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890–900. [DOI] [PubMed] [Google Scholar]
- [2].Hoffman J. The global burden of congenital heart disease. Cardiovasc J Afr 2013;24:141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang Z, Li Z, Ji C. Prevalence study of congenital heart disease in children aged 0-2 in Zhejiang province. Zhonghua Liu Xing Bing Xue Za Zhi 1999;20:155–7. [PubMed] [Google Scholar]
- [4].Shi C, Song L, Li Y, et al. Value of four-chamber view of the fetal echocardiography for the prenatal diagnosis of congenital heart dise. Zhonghua Fu Chan Ke Za Zhi 2002;37:385–7. [PubMed] [Google Scholar]
- [5].Xie D, Yang T, Liu Z, et al. Epidemiology of birth defects based on a birth defect surveillance system from 2005 to 2014 in Hunan province, China. PLoS One 2016;11:e0147280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lie RT, Wilcox AJ, Skjaerven R. A population-based study of the risk of recurrence of birth defects. N Engl J Med 1994;331:1–4. [DOI] [PubMed] [Google Scholar]
- [7].Storch TG, Mannick EE. Epidemiology of congenital heart disease in Louisiana: an association between race and sex and the prevalence of specific cardiac malformations. Teratology 1992;46:271–6. [DOI] [PubMed] [Google Scholar]
- [8].Wu L, Li B, Xia J, et al. Prevalence of congenital heart defect in guangdong province, 2008-2012. BMC Public Health 2014;14:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nikyar B, Sedehi M, Mirfazeli A, et al. Prevalence and pattern of congenital heart disease among neonates in gorgan, Northern Iran (2007-2008). Iran J Pediatr 2011;21:307–12. [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang Y, Riehle-Colarusso T, Correa A, et al. Observed prevalence of congenital heart defects from a surveillance study in china. J Ultrasound Med 2011;30:989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yang XY, Li XF, Lü XD, Liu YL. Incidence of congenital heart disease in Beijing, China. Chin Med J (Engl) 2009;122:1128–32. [PubMed] [Google Scholar]
- [12].Amorim LF, Pires CA, Lana AM, et al. Presentation of congenital heart disease diagnosed at birth: analysis of 29,770 newborn infants. J Pediatr (Rio J) 2008;84:83–90. [DOI] [PubMed] [Google Scholar]
- [13].International Clearinghouse for Birth Defects Monitoring Systems. Proceedings of the 24th annual meeting. Cape town, South Africa, 19 November 1997. Abstracts. Teratology 1998;57:30–44. [DOI] [PubMed] [Google Scholar]
- [14].Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Adv Exp Med Biol 2010;686:349–64. [DOI] [PubMed] [Google Scholar]
- [15].van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241–7. [DOI] [PubMed] [Google Scholar]
- [16].Gray, P. Molecular Motions in Liquids: Proceedings of the 24th Annual Meeting of the Sociètè de Chimie Physique, Paris-Orsay, 2–6 July 1972. (1974): 531. [Google Scholar]
- [17].Annas GJ, Elias S. Thalidomide and the titanic: reconstructing the technology tragedies of the twentieth century. Am J Public Health 1999;89:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li S, Hong S, Wang T. [a population-based surveillance system on birth defects and its application]. Zhonghua Liu Xing Bing Xue Za Zhi 2001;22:172–5. [PubMed] [Google Scholar]
- [19].Favilli S, Santoro G, Ballo P, et al. Prevalence and clinical characteristics of adult patients with congenital heart disease in Tuscany. J Cardiovasc Med (Hagerstown) 2012;13:805–9. [DOI] [PubMed] [Google Scholar]
- [20].Sokal R, Tata LJ, Fleming KM. Sex prevalence of major congenital anomalies in the United Kingdom: A national population-based study and international comparison meta-analysis. Birth Defects Res A Clin Mol Teratol 2014;100:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ou Y, Mai J, Zhuang J, et al. Risk factors of different congenital heart defects in Guangdong, China. Pediatr Res 2016;79:549–58. [DOI] [PubMed] [Google Scholar]
- [22].Cymbron T, Anjos R, Cabral R, et al. Epidemiological characterization of congenital heart disease in Sao Miguel Island, Azores, Portugal. Community Genet 2006;9:107–12. [DOI] [PubMed] [Google Scholar]
- [23].Bosi G, Garani G, Scorrano M, et al. Temporal variability in birth prevalence of congenital heart defects as recorded by a general birth defects registry. J Pediatr 2003;142:690–8. [DOI] [PubMed] [Google Scholar]
- [24].Chen Y, Weng W, Zhang P, et al. Analysis on monitoring results of congenital heart disease of perinatal infants in Bao’an district of Shenzhen City from 2006 to 2010. Chin J Maternal Child Health 2011;26:5780–2. [Google Scholar]
- [25].Jansen FA, Hoffer MJ, van Velzen CL, et al. Chromosomal abnormalities and copy number variations in fetal left-sided congenital heart defects. Prenat Diagn 2016;36:177–85. [DOI] [PubMed] [Google Scholar]
- [26].Guillem P, Fabre B, Cans C, et al. Trends in elective terminations of pregnancy between 1989 and 2000 in a French county (the Isère). Prenat Diagn 2003;23:877–83. [DOI] [PubMed] [Google Scholar]
- [27].Baspinar O, Karaaslan S, Oran B, et al. Prevalence and distribution of children with congenital heart diseases in the central anatolian region, turkey. Turk J Pediatr 2006;48:237–43. [PubMed] [Google Scholar]
- [28].Garne E, Loane M, Dolk H, et al. Prenatal diagnosis of severe structural congenital malformations in Europe. Ultrasound Obstet Gynecol 2005;25:6–11. [DOI] [PubMed] [Google Scholar]


