Figure 1.
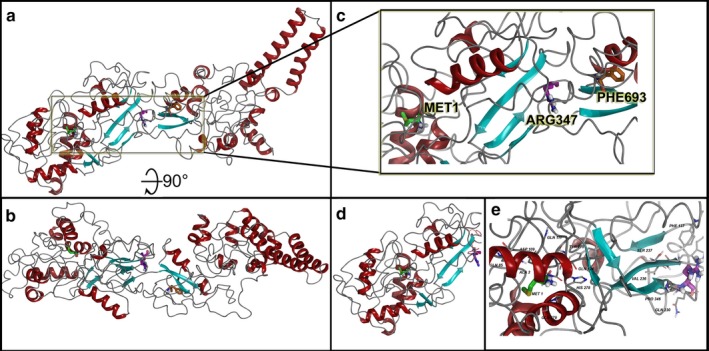
TAB2 molecular model for full‐length human sequence consisting of 693 amino acids and the truncation variant p.R347X. (a) Full‐length model for the entire TAB2 structure that shows two clear domains separated around amino acid 350–360, where R347 plays a role in interacting between the two domains. (b) Rotation of the full‐length model by 90° in X‐axis to better show the domains. (c) Zoom into the region around Arg347 and how the folded protein has Met1 and Phe693 folded within 35Å of each other. (d) Truncated TAB2 at R347X, showing smaller N‐terminus domain lobe. (e) Zoom into the N‐terminus domain lobe with residues within 12 Å of R347 shown and labeled. All protein ribbons are colored by secondary structure and residues shown in licorice rendering and using standard element coloring (C‐gray, O‐red, N‐blue, H‐white, S‐yellow) except for the highlighted residues (Met1‐green carbons, Arg347‐purple carbons, Phe693‐orange carbons)
