Fig. 3. αvβ6 integrin down-regulation in exosomes inhibits recipient monocyte M2 differentiation.
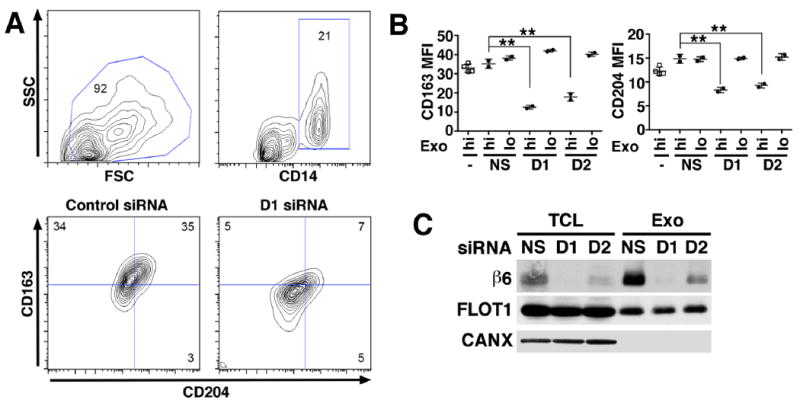
Human PBMC (2.0×105 cells) derived from two healthy donors were incubated for 48 hours with different concentrations of exosomes (Exo) (hi: 8 μg/mL; lo: 0.8 μg/mL) derived from PC3 cells incubated with no siRNA, a non-silencing siRNA (NS) or with one of two different siRNAs specific to β6 mRNA (D1 and D2). Cultures were harvested at 48 hours for M2 monocyte polarization analysis by flow cytometry. (A), Contour plots depict live gating of CD14+ monocytes and representative expression of M2 polarization markers CD163 and CD204 in this population. Numbers indicate the percentage of gated cells. (B), There are 4 different PBMC treatment groups: untreated cells (-); NS, PBMC treated with exosomes from PC3 cells transfected with non-silencing siRNA; D1, PBMC treated with exosomes from PC3 cells transfected with a β6-siRNA duplex designated D1; D2, PBMC treated with exosomes from PC3 cells transfected with a different β6-siRNA designated D2. Mean fluorescence intensity (MFI). The percentages of CD14 gated CD163+/CD204+ cells in D1-hi, and D2-hi are statistically lower than NS, as assessed by Dunnett’s test. **: P > 0.01. (C), IB analysis of β6 integrin expression in TCL and Exo derived from PC3 cells transfected with NS siRNA or with β6 siRNA (D1 and D2). Flotilin 1 (FLOT1) was used as loading control for TCL and Exo, whereas CANX was used as loading control for TCL but not Exo.
