Abstract
A number of infectious, inflammatory and idiopathic lesions develop within otologic tissues that may share similar clinical and/or microscopic features. This review first provides a working classification for otitis externa, and then otitis media and includes two recently described entities, eosinophilic otitis media and otitis media with ANCA-associated vasculitis. Next, the microscopic findings of a spectrum of otopathologic conditions are described, including post-inflammatory conditions such as tympanosclerosis and aural polyps, an overview of animate aural foreign body as well as iatrogenic aural foreign body reactions. Finally, a review of fungal disease affecting the ear with a brief synopsis of Candida auris, a recently described and virulent organism, is presented.
Keywords: Otitis externa, Otitis media, Aural polyp, Tympanosclerosis, Otomycosis, Myospherulosis
Overview
Microscopic classification of infectious, inflammatory, iatrogenic and idiopathic otopathology can be challenging in view of the overlapping and/or unfamiliar clinical terminology of the external and middle ear region, the relative infrequency of ‘normal’ tissue for comparison to pathologic changes and the small size of the anatomic region affected which yields proportionately small sized tissue samples. The clinical findings documented during in-office examination or intraoperatively with the surgical microscope may provide a more informative ‘gross’ examination than the gross pathologic description of small, detached formalin fixed tissue fragments. For these reasons, interdisciplinary communication remains a critical component of patient care. The objective of the current review is to highlight salient clinical and histopathologic features in selected types of otitis externa, otitis media, and associated sequelae such as post-inflammatory, infectious and related foreign body/reactions.
Otitis Externa
Introduction
Otitis externa (OE) refers to a relatively broad group of infectious/inflammatory conditions affecting the external ear and external auditory canal (EAC) that includes acute (diffuse) otitis externa, acute localized otitis externa, chronic otitis externa, necrotizing otitis externa, otomycosis, herpetic otitis and radiation-associated otitis (Table 1). Most times, distinguishing between variants of OE is based clinically upon parameters such as the patient’s signs, symptoms, site of involvement, clinical history and time course of the disease. In some settings, tissue sampling for microscopic examination can facilitate OE classification and/or exclusion of competing entities in the clinical differential diagnosis. With this in mind, the clinical and histologic findings of chronic otitis externa, and necrotizing otitis externa are examined in greater detail with otomycosis discussed in ‘Fungal Diseases of the Ear’ [1].
Table 1.
Classification of otitis externa
| Acute otitis externa |
| Synonyms: acute diffuse otitis externa, hot weather ear, swimmer’s ear |
| Fungal otitis externa possible (otomycosis) |
| Acute localized otitis externa |
| Synonyms: furunculosis, acute circumscribed otitis externa |
| Chronic otitis externa |
| Eczematous otitis externa, subset of chronic otitis externa |
| Necrotizing otitis externa |
| Synonyms: malignant otitis externa, progressive necrotizing otitis, invasive external otitis, skull base osteomyelitis |
| Otomycosis |
| Herpetic otitis externa |
| Radiation-associated otitis externa |
Chronic Otitis Externa
Background
Chronic otitis externa (chronic OE) is a common condition affecting the external ear and EAC. Despite a relatively narrow clinical phenotype, chronic OE is associated with a diverse spectrum of underlying etiologic factors. Chronic OE can occur as a result of idiopathic or autoimmune diseases with the potential for systemic involvement such as amyloidosis, sarcoidosis, or granulomatosis with polyangiitis, localized infectious and/or inflammatory conditions of the external ear (example: unresolved acute bacterial or fungal external otitis) or, as an aural extension of a primary dermatologic condition. The latter encompasses a group of primary dermatologic disorders collectively referred to as eczematous otitis externa (eczematous OE) when identified within the external ear or EAC. The dermatologic conditions most commonly implicated in eczematous OE include atopic, allergic or contact dermatitis (jewelry, lotions or cleaning products, hearing aids, etc.), psoriasis, and lupus [2]. In some patients, active middle ear disease with purulent drainage can macerate the lining of the external canal with cycles of acute and chronic inflammation and result in chronic OE [3].
Compared to acute OE, pain is less often the presenting patient concern in chronic OE. Rather, the patient with chronic OE most often identifies aural discharge and relentless pruritus as being most problematic [2]. Chronic manipulation to relieve itch and/or address aural discharge leads to repeated cycles of excoriation, inflammation, and healing that increases the risk for a superimposed acute infection requiring treatment in order to stabilize the ear. Clinically, chronic OE most often appears as one of two general forms present for at least 3 months in duration: a scaly, dry ear with hypertrophic tissues and possible scattered erythematous areas along canal skin, or as a moist, oozing, edematous, erythematous, fissured ear and canal. Physical exam findings in chronic OE vary depending on the underlying etiology, and whether or not an acute exacerbation is present. Chronic OE can be unilateral or bilateral [2]. Since the clinical appearance of chronic OE represents a common pathway for one or more underlying diseases, additional testing such as tissue sampling, may be helpful in delineating the etiology.
Histopathologic Findings
Biopsy sampling of the affected tissues are evaluated for the presence of chronic granulomatous inflammation, superficial dermatophyte infection, a spectrum of inflammatory dermatopathology including subacute and chronic spongiotic dermatides, and reactive changes such as hyperkeratosis, hypergranulosis, reactive epithelial hyperplasia (pseudoepitheliomatous hyperplasia), acanthosis and papillary dermal fibrosis. Surgeons are keenly aware that in situ or invasive squamous cell carcinoma occurring within the ear canal can clinically mimic chronic OE and submit tissue samples to evaluate for the presence of malignancy. Distinction between a well-differentiated squamous carcinoma and small tangentially oriented fragments of reactive epithelial hyperplasia (pseudoepitheliomatous hyperplasia) with adjacent inflammation may represent a diagnostic challenge in some cases.
Treatment
Treatment of chronic OE is based on identifying the underlying causes where possible, eliminating predisposing or exacerbating factors and educating the patient on aural care to optimize local conditions and avoid local irritation [2, 3]. Though material from the canal can be submitted for culture if bacterial or fungal infection is suspected, results may be inconclusive in a patient who has been using topical or oral antibiotic therapy [1]. Over time, chronic OE results in hypertrophy of the EAC skin and subepithelial tissues that narrow the lumen of the external ear canal (acquired canal fibrosis) contributing to conductive hearing loss [1]. Aside from biopsy sampling, surgery is rarely indicated for chronic OE unless surgery to remove medial canal fibrosis is being considered [2].
Necrotizing Otitis Externa (NOE)
Background
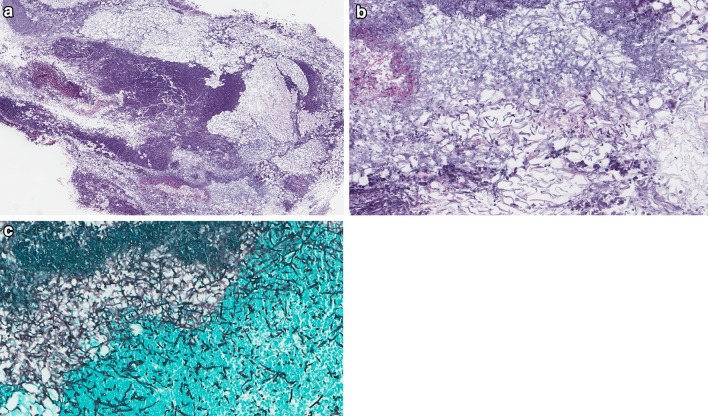
Necrotizing (malignant) otitis externa is an aggressive, potentially life threatening infection occurring at the junction of the osseous and cartilaginous external auditory canal that can clinically resemble acute OE in its early stages [4]. Infection and inflammation spread from the osteocartilaginous junction or osseous canal along various anatomic routes; posteriorly toward the mastoid process, anteriorly/inferiorly via the fissures of Santorini (Fig. 1a–e) toward the temporomandibular joint, parotid and cervicofascial spaces or medially into the skull base/petrous apex. The first English language description is credited to Meltzer in 1959 [5], followed by a more complete understanding of the disease in 1968 by Chandler, who coined the term malignant otitis externa in view of the high rate of disease specific mortality at this period of time [6]. Subsequently, Evans and Richards introduced the term necrotizing otitis externa (NOE) [7]. Malignant otitis externa and necrotizing otitis externa are terms that have endured over time in the literature and both are used and accepted [8]. The term NOE is used in the remainder of this discussion.
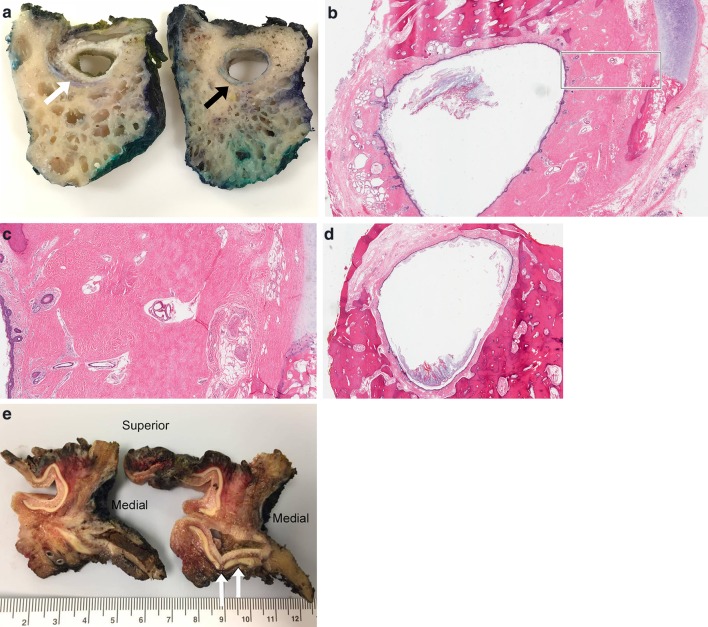
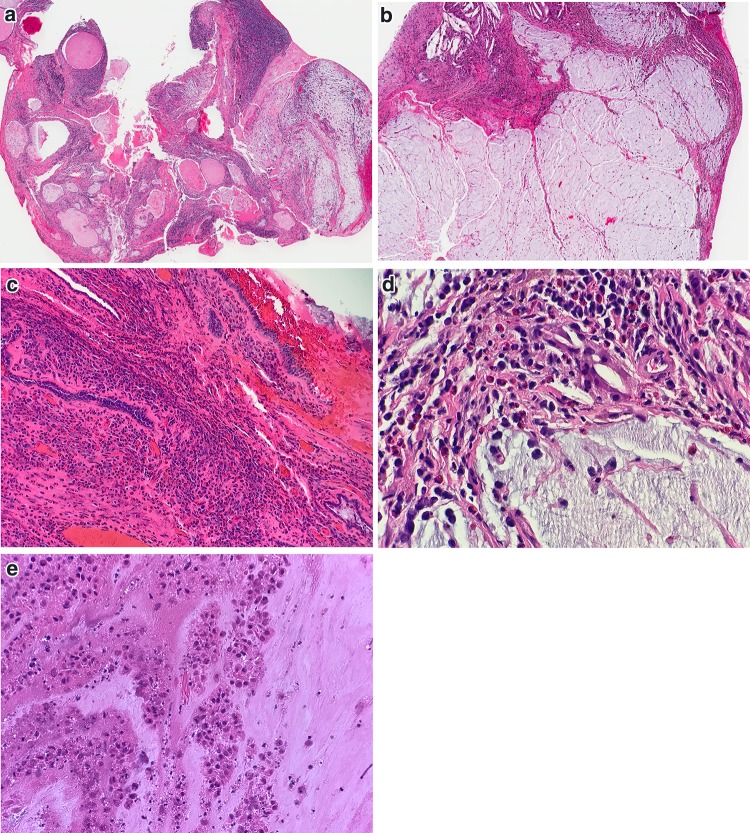
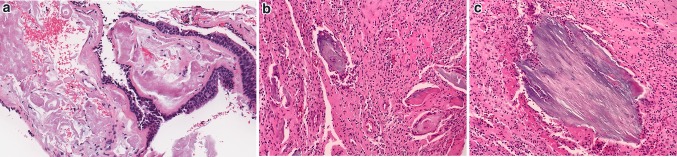
Fig. 1.
Gross and microscopic examination of ear and temporal bone anatomy. a Cross section of temporal bone resection showing cartilaginous EAC (white arrow) and osseous EAC (black arrow). b Histologic cross section through region of osteocartilaginous junction of EAC. Black box, see c (Hematoxylin and eosin [H&E], 11x). c Low power magnification of normally dense fibrous connective tissue at osteocartilaginous junction (H&E, 25x). d Histologic cross section through osseous EAC (H&E, 12x). e Composite resection sectioned lateral to medial through external ear, long axis of EAC and surrounding tissues resected for infiltrating squamous cell carcinoma. White arrows highlight fissures of Santorini
NOE predominantly affects elderly patients, and patients with diabetes or other conditions resulting in immunodeficiency [4, 9–11]. It has been suggested that microangiopathy or altered immune function increases the risk of developing infection or perpetuates infection in the area [12, 13]. Men are more often affected by NOE.11 Classically, the infectious agent most commonly associated with NOE is Pseudomonas aeruginosa but other bacterial and fungal agents may be seen [10, 13, 14]. In one series, the second most common pathogen identified after bacteria, was fungi (Aspergillus sp. or Candida sp.) in over a third of patients [10]. Culture data is critical to distinguish fungal from bacterial infection, and also to identify organisms with multidrug resistance.
The symptoms and clinical presentation of NOE consist of a unilateral and unremitting, severe otalgia that radiates to the jaw and frequently is nocturnal [10, 12]. Headache may be present but fever, tachycardia and other signs of severe infection are often absent. On clinical examination, otorrhea, edema of the EAC, and ulceration of the skin and formation of granulation tissue at the osteocartilaginous portion of the canal may be identified [12]. Cranial nerve palsies, most commonly affecting the facial nerve, occur later in the disease process [9, 11]. Depending on the examination findings, acute OE, acute exacerbation of chronic OE, otomycosis, foreign body, osteomyelitis and malignancy such as squamous cell carcinoma may fall within the clinical differential diagnosis of NOE. With imaging, computed tomography (CT) detects bone erosion in the canal and skull base, formation of abscess collection and extension into adjacent structures. A negative CT scan does not exclude NOE, as it is possible that early minimal bone destruction may be undetectable in the initial phase of the disease [8–10].
Histopathologic Findings
In health, a thin layer of stratified squamous epithelium lines the dense fibrous connective tissue layer along the osseous EAC. Dense subepithelial fibrous tissue is present at the osteocartilaginous junction and regions of the osseous canal (Fig. 1c, d). In patients with NOE, biopsy samples taken from the osseous EAC area may reveal ulceration/loss of the overlying epithelium, with bacteria and inflammation extending into the dense fibrous connective tissue layer (Fig. 2a, b) [15]. Where canal epithelium remains intact, reactive changes ranging from mild hyperplasia to prominent pseudoepitheliomatous hyperplasia are present. Marked, and often full thickness acute and chronic inflammation including abscess formation is common (Fig. 2c, d). In biopsy samples overlying the cartilaginous canal, inflammation extends into the apopilosebaceous apparatus. Reactive capillary proliferation is evident within the granulation tissue [16]. Non-viable bone fragments with associated inflammation may be present for evaluation. Chronic granulomatous inflammation with granuloma formation is not associated with NOE. Identification of micro-organisms (bacterial/fungal) is often augmented by special stains performed on tissue. The extent of the work up may be influenced by concomitant culture material results and whether organisms have been identified. In one study, nearly a third of cultures failed to show growth [14]. In culture-negative NOE, the Pathology team may be consulted in select cases to evaluate the utility of pursuing testing such as a polymerase chain reaction (PCR) assay on tissue samples in order to maximize the possibility of detecting fungal or bacterial elements [4].
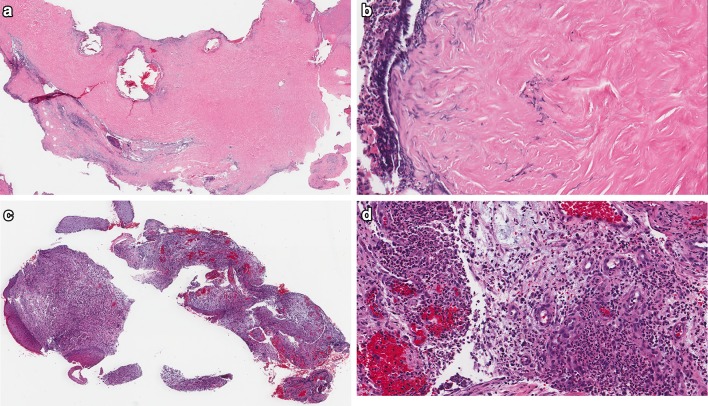
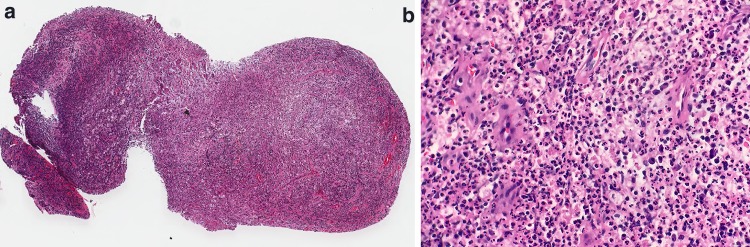
Fig. 2.
Spectrum of microscopic changes in necrotizing otitis externa. a Dense fibrous connective tissue at osteocartilaginous junction of EAC, devoid of surface epithelium (H&E, 16x). b Higher magnification of a, with bacterial colonies and inflammation permeating dense connective tissue (H&E, 200x). c Biopsy sample of clinical ‘granulation tissue’ within EAC in patient with NOE (H&E, 26x). d Edematous, markedly inflamed granulation tissue in NOE within EAC (H&E, 120x)
Differential Diagnosis
Pseudoepitheliomatous hyperplasia admixed with marked inflammation can be challenging to differentiate from very well differentiated squamous carcinoma. The presence of NOE does not exclude the possibility of invasive carcinoma, as these diseases have been reported to occur concurrently [17]. In the setting of prior radiation therapy for malignancy, the presence of inflammation, granulation tissue and non-viable inflamed bone could appear similar to osteoradionecrosis. Grocott’s methenamine silver for fungal organisms (GMS) or periodic acid-Schiff (PAS) can be employed to evaluate for the presence of fungal forms.
Treatment
Management of NOE consists of local wound care, systemic antibiotic treatment ideally guided by culture data and sensitivities, and where applicable, control of blood glucose. Surgery is reserved for local debridement or diagnostic procedures such as deep tissue sampling for biopsy and/or culture [4]. Antifungal therapy is based upon detection of fungal organisms [10]. NOE readily involves the underlying bone and most agree that the duration of antibiotic treatment should approach 6 weeks at least, similar to other forms of osteomyelitis.
Otitis Media
Introduction
Otitis media (OM) is a non-specific term that refers to a spectrum of diseases affecting the middle ear most commonly including acute otitis media, chronic otitis media with effusion and chronic suppurative otitis media [18]. In most cases, the features of acute otitis media and chronic otitis media with effusion can be recognized clinically and managed without the need for histologic evaluation of tissue. In chronic suppurative otitis media, the diagnosis and management may be impacted by histologic findings such as the presence of a specific infection or cholesteatoma among other possible findings. Rare forms of otitis media such as eosinophilic otitis media and otitis media with ANCA-associated vasculitis (OMAAV) may mimic clinical features of chronic suppurative OM but have microscopic findings to allow distinction from chronic suppurative OM which are further discussed in this section (Table 2).
Table 2.
Classification of otitis media
| Acute otitis media |
| Chronic otitis media |
| Chronic otitis media with effusion |
| Chronic suppurative otitis media |
| Eosinophilic otitis media |
| Otitis media with ANCA-associated vasculitis |
Chronic Suppurative Otitis Media
Background
Chronic suppurative OM is a chronic, inflammatory process affecting the middle ear and/or mastoid cavity with persistent or recurrent ear discharge entering the EAC through a defect in the tympanic membrane (TM) [18]. In addition to otorrhea, otalgia and hearing loss may be present. Transitioning to a clinical diagnosis of chronic OM from an acute episode is dependent on time, though the exact timing is a matter of debate and ranges from several weeks to several months. Chronic suppurative OM affects millions of children and adults worldwide and the process can result in damage to middle and inner ear structures, leading to hearing loss and in rare instances, intracranial complications [18, 19]. Many factors play a role in the development and/or perpetuation of chronic suppurative OM including those related to the pathogen, environment, local anatomy, host genetics, immunity and host response but further analysis is beyond the scope of this review [20, 21]. Diagnostic modalities for OM rely on patient history, symptoms, specialized in-office examination techniques to evaluate the TM and middle ear, as well as imaging, intraoperative examination and in many cases, supportive histologic findings.
Histopathologic Findings
Histologic findings will be influenced by the disease state and signs of a chronic, or active-chronic process can lead to changes in the middle ear epithelium, the subepithelial tissues and/or sampled bone. The mucosa of the middle ear cleft is normally surfaced by low cuboidal epithelium and underlying delicate connective tissue, essentially a mucoperiosteum lining bony cavity walls with few or rare goblet intraepithelial cells and normally devoid of glands (Fig. 3a). In response to inflammation, the middle ear epithelium becomes more reminiscent of the lining of the rest of the respiratory tract with the proliferation of goblet cells, columnar cells and in some cases epithelial hyperplasia [20]. Glandular transformation or glandular metaplasia of the epithelium occurs, also referred to as otomastoiditis cystica and glandularis [22]. This inflamed, altered epithelium with glandular metaplasia invaginates into the underlying stroma and imparts the appearance of haphazardly distributed glands separated by variably inflamed fibrotic or edematous stromal tissues. Regions of ciliated epithelium may be identified and the cuboidal middle ear epithelium may or may not be seen (Fig. 3b–c) [23]. Glands may be empty or contain mucoserous secretions (Fig. 3b–f). The presence of stratified squamous epithelium identified within a specimen labeled ‘middle ear contents’ is viewed as abnormal. Stratified squamous epithelium may represent squamous metaplasia, migration of epithelium from the external ear or cholesteatoma. In addition, small detached segments of squamous epithelium could also represent the damaged TM remnant that has been surgically removed and included in the submitted sample. The primary concern regarding the presence of keratinizing stratified squamous epithelium is determining the presence of cholesteatoma, making clinical correlation absolutely essential [24]. There is variation in the degree of inflammation and the infiltrate is composed of acute and/or chronic inflammation with lymphocytes, plasma cells, foamy histiocytes and occasional eosinophils. Inflamed middle ear mucosa with granulation tissue forming a clinically polypoid silhouette exists along a continuum with aural polyp (see Aural (Otic) Polyp). In addition to aural polyps, other incidental histologic findings may be observed in middle ear tissues of chronic suppurative OM including cholesterol granuloma, foreign body reaction to surgical materials, tympanosclerosis (see Tympanosclerosis), keratin granuloma, cholesteatoma and rarely, mucosal associated lymphoid tissue (Fig. 3g, h) [24, 25].
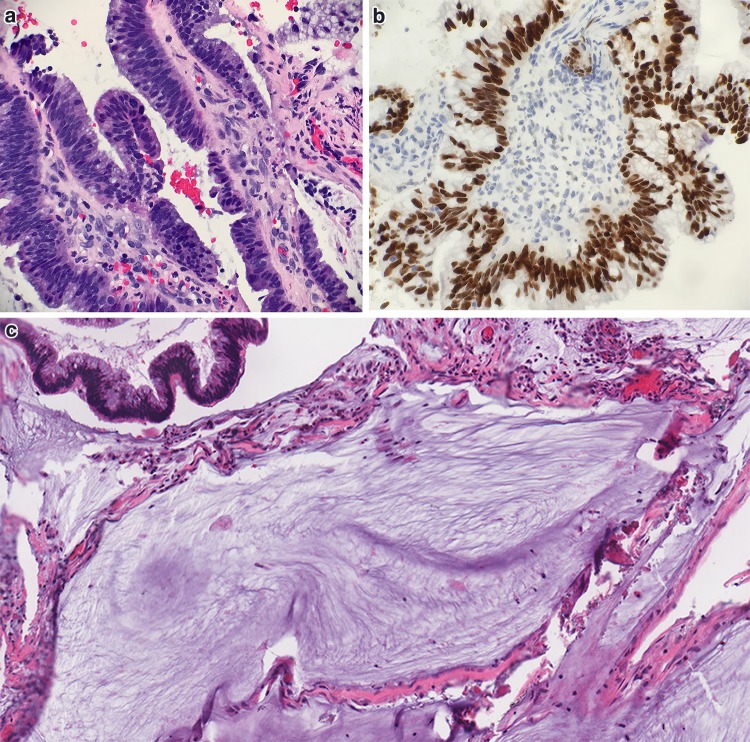
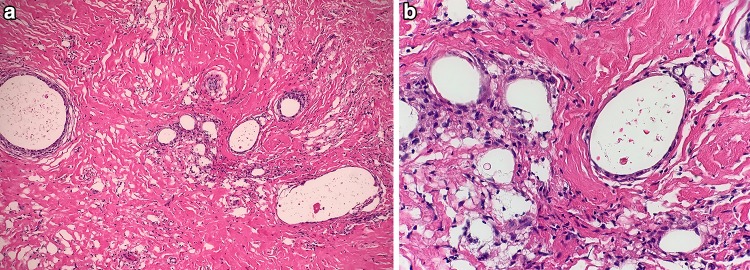
Fig. 3.
Otitis media. a Low cuboidal middle ear epithelium and delicate fibrous connective tissue overlying bone in uninvolved middle ear (H&E, 200x). b–d Dense fibrous connective tissue with glandular metaplasia in OM (H&E, 40x). c Fibrous connective tissue between glandular structures (H&E, 100x). d Glandular metaplasia with seromucous secretions (H&E, 200x). e–f Glandular metaplasia set in edematous, granulation type middle ear stroma (H&E 100x). f Higher magnification of glandular metaplasia (H&E, 200x). g Cholesterol clefts within cholesterol granuloma (H&E, 200x). h Foreign body reaction to keratinous debris in keratin granuloma (H&E, 200x). i Uninflamed middle ear ossicle (H&E, 130x)
Not uncommonly, fragments of mastoid/temporal bone, and ossicular tissue may be submitted along with middle ear contents for microscopic examination. Normally, the mineralized ossicles are composed of dense lamellar bone with peripheral areas of cartilage surrounded by fibrous tissue and low cuboidal epithelium of the middle ear (mucoperiosteum) (Fig. 3i). Bony erosion, remodeling and inflammation of ossicle occurring in the course of chronic OM may appear histologically similar to osteomyelitis, however the bone damage is usually limited to the delicate ossicles and treated with local measures. A search for the cartilaginous rim of ossicular tissue, along clinical correlation will assist in this distinguishing ossicular bone inflammation/damage from cases of true temporal bone osteomyelitis in most cases.
Differential Diagnosis
Middle ear glandular metaplasia can mimic middle ear adenoma, a gland forming neuroendocrine neoplasm. In the latter, there is more variability and complexity of the epithelial elements such as the formation of nests, cords, trabeculae, tubules and gland formation (Fig. 4a). Synaptophysin positive, cytokeratin AE1/3 positive tumor cells of middle ear adenoma are plasmacytoid with a fine chromatin pattern. Identification of cilia would not be expected in the glandular proliferation of middle ear adenoma. Repeated cycles of inflammation and connective tissue fibrosis can result in reactive middle ear tissues composed of cellular stromal hyperplasia interspersed by glandular metaplasia (Fig. 3b–f), an arrangement that can mimic a recently described middle ear neoplasm, mixed epithelial and stromal tumor of middle ear (MESTME) [26]. MESTME is characterized by multiple bland epithelial cysts set within a cellular, ovarian-type stroma. Thus far, the immunohistochemical profile is non-specific. The reporting authors determined MESTME to be neoplastic on the basis of monoclonality, but too few cases are available to be certain of biologic potential [26]. In view of the hypercellularity of exuberantly inflamed, polypoid granulation tissue from the middle ear, one must remain vigilant for subtle clues of middle ear neoplasms that can mimic inflammatory or reactive tissue changes. For example, small crushed islands of middle ear paraganglioma may be inconspicuous within the inflamed fibroglandular and vascular stromal tissues and can be overlooked if areas of better preserved tumor are not identified (Fig. 4b–e). Similarly, the small hyperchromatic cells of embryonal rhabdomyosarcoma or the neoplastic cells of Langerhan’s Cell Histiocytosis can be difficult to identify on routine stained sections alone and use of ancillary testing may be required to exclude these diseases in some cases. Aside from Langerhan’s Cell Histiocytosis, the presence of numerous histiocytes, particularly when arranged in a sheet-like pattern with granular to vacuolated cytoplasm (Hansemann cells) has been described in aural malakoplakia, an extremely rare reactive process in the middle ear that represents a host reaction to an infectious process, most commonly bacteria. The presence of small calcified bodies with a targetoid appearance known as Michalis-Gutman bodies show reactivity to Prussian blue (iron) and Von kossa (calcium) staining [27].
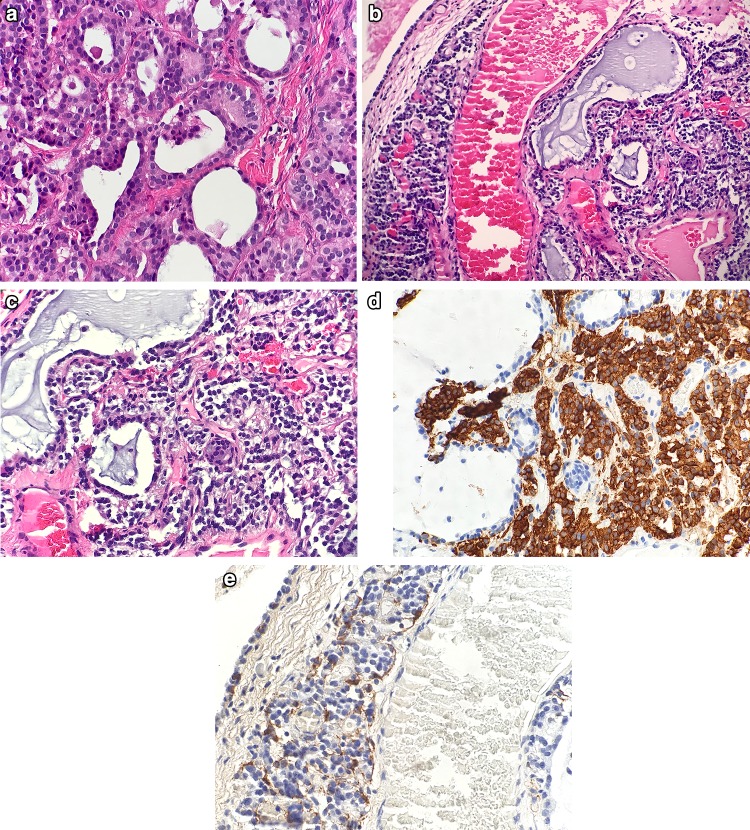
Fig. 4.
Differential diagnosis of Otitis Media. a Back to back glands of middle ear adenoma (H&E, 200x). b Middle ear paraganglioma surrounding inflamed middle ear tissue with glandular metaplasia (H&E, 100x). c Middle ear paraganglioma (H&E, 300x). d Synaptophysin immunostain positive in middle ear paraganglioma (300x). e S100 immunohistochemical stain highlights sustentacular population in middle ear paraganglioma (400x)
Treatment
A combination of local/topical therapy, aural toilet and management of the middle ear granulation tissue and mucosa form the treatment foundation of chronic suppurative OM. Surgical intervention is almost always indicated [21].
Eosinophilic Otitis Media (EOM)
Background
The term EOM was introduced over 20 years ago by Tomioka et al. [28] and is used to describe a separate and distinct form of OM. EOM exhibits a viscous, eosinophil-rich middle ear mucoid effusion, in which the majority of cases are bilateral and refractory to standard OM treatment [29]. In addition, EOM is a disease that primarily occurs in patients with a pre-existing history of bronchial asthma alone or a combination of bronchial asthma, and nasal polyposis with many patients also reporting aspirin intolerance (aspirin exacerbated respiratory disease). EOM is clinically significant in view of the risk of hearing loss, a risk much higher than expected in classic otitis media [29]. EOM occurs in adults between the ages of 50–60, with a slight female predilection [30]. Symptoms include otorrhea, tinnitus and diminished hearing. In most cases the disease is bilateral, although the condition is of the middle ear mucosa and is not necessarily identical between the two sides [30, 31]. A viscous, yellow-tinged mucoinflammatory effusion is present [30]. Hearing loss may be conductive in nature due to the formation of middle ear eosinophilic aural polyps that extend into the external auditory canal, along with the viscous mucoinflammatory otorrhea, although some patients may have sensorineural hearing loss or mixed hearing loss [31–33].
Association of EOM with Aspirin-Exacerbated Respiratory Disease (AERD)
AERD, formerly Samter’s triad, is constellation of bronchial asthma, nasal polyposis and aspirin sensitivity [34]. Otologic symptoms do not uniformly occur in AERD, but when present, the symptoms develop approximately 5 years after the onset of nasal symptoms. The development of otopathology may be influenced by the degree of AERD disease control [34]. Since the mucoinflammatory otorrhea and eosinophilic aural polyps appear histologically similar to mucoinflammatory sinonasal secretions and sinonasal polyp material it has been proposed that the ‘one disease, one airway’ model may not only apply to sinonasal and bronchial mucosa, but should be extended to include the middle ear mucosa as well [32].
Histopathologic Findings
The fibroglandular tissue aggregates of EOM appear as dense fibrous and granulation tissue punctuated by dilated glands and/or mucin-rich pools. Low cuboidal or ciliated pseudostratified columnar epithelium with goblet cells line the middle ear mucosa and invaginate into the stroma [31]. Subepithelial tissues are inflamed, most often an acute and chronic eosinophil-rich inflammatory response is identified [35]. Eosinophils and Charcot-Leyden crystals are present within detached mucoid debris or extracellular mucous identified dissection within middle ear tissues (Fig. 5a–e) [31]. Aural polyps with prominent eosinophilic inflammation may be present. No granulomatous inflammation or tissue necrosis is seen. The diagnostic criteria for EOM established in 2011 represents a combination of clinical and histologic findings (Table 3) [29].
Fig. 5.
Eosinophilic otitis media (EOM). a Scanning magnification of multicystic inflamed middle ear mucosa with extracellular mucin and granulation tissue (H&E, 12x). b Extracellular mucin dissects inflamed fibrogranulation tissue of middle ear (H&E, 30x). c Prominent tissue eosinophilia within fibrogranulation tissue (H&E, 200x). d Tissue eosinophilia and extracellular mucin (H&E, 400x). e Detached mucoinflammatory debris submitted from middle ear with Charcot–Leyden crystals present (H&E, 400x)
Table 3.
Diagnostic criteria of eosinophilic otitis media (EOM)
| Major criteria: otitis media (OM) with effusion or chronic otitis media with eosinophil-rich effusion |
| Minor criteria |
| 1. Highly viscous middle ear effusion |
| 2. Refractory to conventional treatments for OM |
| 3. Association with bronchial asthma |
| 4. Association with sinonasal polyposis |
| Definitive case: positive for major and at least two minor criteria |
| Exclusion criteria: eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss syndrome), hypereosinophilic syndrome |
Adapted from Reference [29]
Differential Diagnosis
Mucinous head and neck neoplasms such as mucoepidermoid carcinoma or intestinal-type adenocarcinoma may enter diagnostic consideration in view of the pools of mucin within tissues. In contrast to reactive middle ear epithelium with glandular metaplasia, mucoepidermoid carcinoma exhibits irregularly shaped nests or cystic structures composed of epidermoid, intermediate and mucous cells in varying proportion. Pools of mucin may also be seen in association with sinonasal intestinal-type adenocarcinoma. A large sinonasal intestinal-type adenocarcinoma can secondarily invade into the middle ear or external auditory canal, and at least one case of primary intestinal-type adenocarcinoma of the middle ear has been reported [36]. The appearance of neoplastic intestinal-type epithelium can be distinguished from middle ear mucosa by the elongated, hyperchromatic, and often crowded appearance of the epithelium (Fig. 6a–c). Given the microscopic similarity between EOM and sinonasal allergic fungal sinusitis, GMS special staining may be employed to evaluate for fungal elements. EOM is expected to be negative for fungal forms and detection of fungal elements within the mucoid debris may suggest a different clinical spectrum from that of true EOM. For example, patients with fungal elements identified on GMS staining of middle ear allergic-type/eosinophilic mucin are more likely to have unilateral chronic ear disease, but absent any prior history of bronchial asthma or even sinonasal polyposis, which stands in sharp contrast to EOM [37, 38].
Fig. 6.
Differential diagnosis of EOM. a Intestinal-type epithelium overlying granulation tissue submitted as clinical “polyp” from region of tympanic membrane. Patient had large infiltrative sinonasal intestinal type adenocarcinoma resected from ethmoid sinuses 6 months prior (H&E, 100x). b CDX2 immunohistochemical staining of intestinal type epithelium (400x). c Mucoid material associated with sinonasal intestinal type adenocarcinoma infiltrating into middle ear (H&E, 70x)
Treatment
The optimal treatment plan is still being elucidated but currently involves topical and systemic steroids, use of antibiotics (where indicated), and in some severe cases, anti-IgE treatment such as omalizumab [30]. Control of underlying systemic pulmonary disease and/or aspirin desensitization can have a role for some patients [32, 39]. Removal of polyps may be indicated in some cases but other traditional surgical approaches are unlikely to control symptoms for long. The major risks with untreated EOM include progression to severe sensorineural hearing loss, and intracranial inflammation or infection [40]. The role of bacteria in EOM is yet to be clarified, and culturing of material may be warranted [30, 41].
Otitis Media ANCA-Associated Vasculitis (OMAAV)
Background
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a disease known to affect multiple organ systems and is characterized by necrotizing inflammation of small or medium-sized blood vessels. Three subtypes of AAV are recognized; granulomatosis with polyangiitis (formerly Wegener’s granulomatosis), eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss syndrome) and microscopic polyangiitis [30, 42]. Aside from kidney and lung involvement, AAV is known to affect subsites in the head and neck. Recently, it has been proposed that AAV may present clinically as otitis media refractory to treatment, termed otitis media with anti-neutrophil cytoplasmic antibody associated vasculitis (OMAAV) [42]. When symptoms are localized to the ear, early and accurate diagnosis can be challenging.
Symptoms overlap with other forms of chronic otitis media such as otorrhea, tinnitus, otalgia and mild hearing loss and the process can be unilateral or bilateral [42]. OMAAV occurs most commonly in women over a wide age range, with a peak reported in the seventh decade of life [42–44]. In the latter population, a recent history of adult onset of ear symptoms in a patient without a history of prior ear problems, particularly when bilateral would be an unusual presentation for classic chronic otitis media. Neurologic findings include vertigo, hypertrophic pachymeningitis and/or facial nerve weakness. In advanced cases, complete bilateral hearing loss is reported. On imaging the middle ear and/or mastoid cavity exhibits opacification, most often without evidence of bone erosion [43].
Histopathologic Findings
The low cuboidal middle ear epithelium may be present or epithelial hyperplasia, with mucous cell metaplasia identified. Subepithelial tissues can appear fibrotic, or as edematous granulation tissue, and acute and chronic lymphoplasmacytic inflammation is present. Giant cells and granuloma formation have been described but are not required features [45]. ‘Blue necrosis’ (geographic tissue necrosis) or collagenous necrosis is often seen (Fig. 7a, b) [43]. The pools of eosinophilic mucin and tissue eosinophilia seen in EOM are absent in OMAAV.
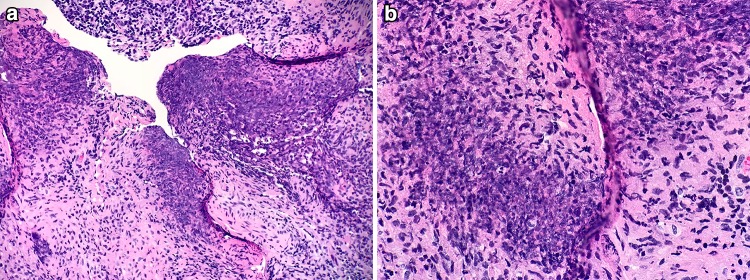
Fig. 7.
Otitis Media with ANCA-associated vasculitis. a Middle ear fibrogranulation tissue with exuberant inflammation, crush artifact and tissue necrosis (H&E, 100x). b Higher magnification of tissue necrosis (H&E, 200x). ANCA anti-neutrophil cytoplasmic antibody
Differential Diagnosis
Exuberant chronic otitis media (COM) could share some histologic similarities to OMAAV but would not be expected to show tissue necrosis or granulomatous inflammation. Identification of stromal fibrosis with lymphoplasmacytic inflammatory infiltrate may raise consideration of IgG4 related-sclerosing disease, but with few IgG4 plasma cells present in OMAAV the finding remains non-specific. The presence of chronic granulomatous inflammation prompts a broader differential diagnosis to include fungal infection, tuberculous otitis, sarcoidosis, foreign body, and otosyphilis. GMS and Acid Fast Bacillus (AFB) special stains should be employed but must be negative for organisms in OMAAV. The diagnostic criteria of OMAAV are delineated in Table 4.
Table 4.
Diagnostic criteria for otitis media with ANCA-associated vasculitis (OMAAV)
| OMAAV classified if the following three criteria fulfilled |
| 1. Disease onset with intractable otitis media (OM) resistant to conventional treatment |
| 2. At least one of the following |
| Serum positive for MPO- or PR3-ANCA |
| Histopathology consistent with ANCA associated vasculitis (necrotizing vasculitis predominantly affecting small vessels with or without granulomatous inflammation) |
| At least one accompanying sign or symptom of ANCA associated vasculitis related organ or tissue involvement other than the ear (eye, nose, pharynx, larynx, lung, kidney, facial palsy, hypertrophic pachymeningitis) |
| 3. Exclusion of other types of intractable OM and other autoimmune diseases with vasculitis |
| Bacterial otitis media, cholesterol granuloma, cholesteatoma, skull base osteomyelitis, tuberculous otitis, neoplasm, eosinophilic otitis media |
| Exclusion of other autoimmune diseases with vasculidities (ex: Cogan’s syndrome and polyarteritis nodosa |
Adapted from Reference [43]
Serologic Testing
In patients with OMAAV, the most commonly reported positive serologic finding is MPO-ANCA. This is followed by a roughly equal proportion of patients exhibiting either PR3-ANCA positivity, or patients negative for MPO-ANCA and for PR3-ANCA (‘double-negative’). Some patients with double-negative ANCA status may over time develop positive ANCA serology [43].
Treatment
It remains unclear whether OMAAV is best considered an otologic manifestation of systemic AAV, a unique subtype of AAV or a unique otopathologic entity, and these uncertainties make the treatment of OMAAV controversial. The disease is rare therefore more challenging to study, and there can be difficulty in confirming the diagnosis in some cases, particularly in patients negative for both MPO and PR3 ANCA. Once the diagnosis is established, most recommend management with corticosteroids and immunosuppressive agents [42, 43] as well as interdisciplinary otolaryngology/neuro-otology, neurology, pulmonology, rheumatology, urology, and ophthalmology evaluation [42]. Mortality associated with OMAAV is rare, but has been reported due to the development of subarachnoid hemorrhage [43].
Otologic Post-inflammatory Sequelae
Tympanosclerosis
Background
Tympanosclerosis (TS) is a common post-inflammatory condition affecting the middle ear and tympanic membrane (TM) that occurs in response to chronic otitis media, chronic infection of the middle ear and/or trauma (Table 5) [46]. When only the TM is affected, the condition is termed myringosclerosis [47]. Although the clinical and histomorphology of TS are known, the etiopathogenesis is not fully established. One hypothesis describes a chronically inflamed, hypoxic environment which leads to the production of free oxygen radicals, tissue injury and subsequent hyaline degeneration and calcification [46]. Damage to inflamed middle ear mucosa compromises function with subsequent impaired movement of inflammatory and/or seromucoid exudate. Over time, maturation results in organization and sclerosis of tissues [48]. TS is a condition affecting patients older than 30 years of age, and the reported frequency varies from 3 to 62.9% and the broad range may be related to severity, extent of disease and method of assessment (clinical vs histologic confirmation) [46, 48]. TS can be an important clinical disorder when it results in impairment of sound transmission due to scarring and ossicular fixation [48–50]. Clinical examination reveals white semicircular or crescent shaped plaques which vary in consistency depending on the degree of calcification on the TM and clinically may show similarity to cholesteatoma in some cases [51, 52]. In the middle ear cleft, granulation tissue and thickened mucous membranes exhibit small punctate foci, or tan/white plaques of sclerotic tissue. Multiple sites (TM, middle ear and less commonly mastoid process) are involved in over 30% of cases [50].
Table 5.
Selected sequelae of external otitis and/or otitis media
| Acquired stenosis of external auditory canal |
| Secondary cholesteatoma |
| Tympanosclerosis |
| Aural (otic) polyps |
| Foreign body reaction |
| Cholesterol granuloma |
| Keratin granuloma |
| Myospherulosis |
| Infection: bacteria, fungus, mycobacteria, others |
Histopathologic Findings
Thick, often curvilinear hyalinized bands are present within the subepithelial connective tissues, along with scattered granular deposits of dystrophic calcifications or areas of frank ossification [48, 52]. The surrounding mucosa exhibits granulation tissue, acute and/or chronic inflammatory cells and reactive changes in the middle ear epithelium such as epithelial hyperplasia or metaplasia (Fig. 8a–c) [52]. Associated findings in the submitted specimen may include cholesteatoma, cholesterol granuloma, or fragments of damaged tympanic membrane [48, 50]. Normally, the pars tensa of the TM is lined along its lateral surface by stratified squamous epithelium, and the medial surface by a low cuboidal, simple epithelium (Fig. 8d). The collagenous zone positioned between these epithelial surfaces consists of a two layered fibrous matrix with radially arranged collagenous fibers toward the lateral aspect and a medial layer in which fibers are arranged circularly. This eosinophilic, band-like layered fibrous matrix in the TM may be the site of dystrophic calcifications or ossification in myringosclerosis possibly obscuring microscopic recognition of the tissue as TM (Fig. 8e, f).
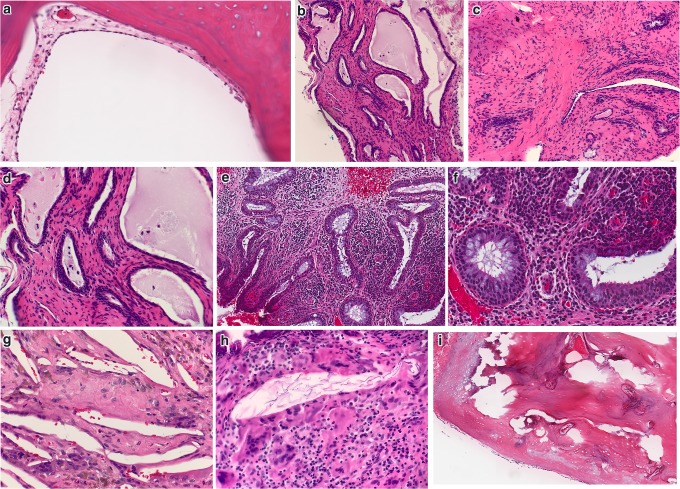
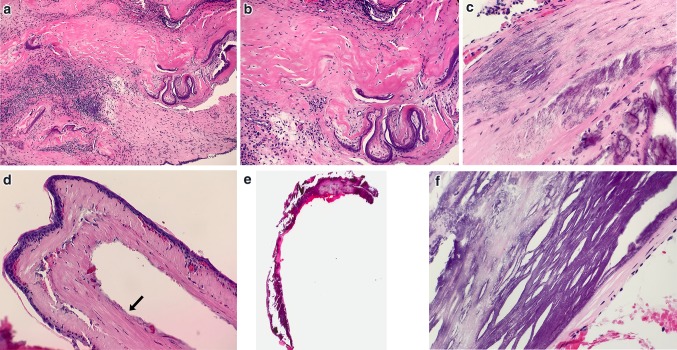
Fig. 8.
Tympanosclerosis. a Middle ear mucosa with stromal hyalinization and calcification of tympanosclerosis (H&E, 100x). b Higher power magnification of hyalinization and calcification (H&E, 200x). c Developing linear and stippled calcifications (H&E, 300x). d Normal tympanic membrane (TM), layering of lamina propria is evident. Black arrow indicates expected region of cuboidal middle ear mucosa, not in view (H&E, 20x). e Scanning magnification of TM with extensive myringosclerosis (H&E, 10x). f Higher magnification of myringosclerosis (H&E, 400x)
Differential Diagnosis
Pathologic processes with microscopic findings similar to TS include the eosinophilic deposits of amyloid (Fig. 9a). Unlike amyloid, the hyalinization of TS does not deposit around submucosal vascular channels, highlight with Congo Red, or exhibit apple green birefringence under polarized light exam. Where marked acute and chronic inflammation has disrupted the middle ear mucosa, disruption of the calcified TS fragments can perpetuate an inflammatory response including foreign body giant reaction (Fig. 9b, c). In these cases, distinguishing TS calcification from that of exogenous calcified material related to a prior procedure/graft placement in the middle ear or eliminating the possibility of underlying temporal bone infection or osteonecrosis may be challenging [53].
Fig. 9.
Differential Diagnostic considerations for Tympanosclerosis. a Eosinophilic deposits of amyloid in eustachian tube (H&E, 200x). b Acute infection of middle ear mucosa with suppurative inflammation disrupting calcified plaques of tympanosclerosis (H&E, 100x). c Acute inflammation surrounding disrupted calcifications (H&E, 300x)
Treatment
Treatment of TS occurs in the setting of treating underlying chronic otitis media and often involves the removal of inflamed, hyperplastic middle ear mucosa with TS plaques. Methods of TS prevention have not been fully established [47].
Otic (Aural) Polyps
Background
An aural polyp (AP) represents a polypoid, non-neoplastic proliferation of inflamed granulation tissue arising from damaged middle ear mucosa that may be lined in part with epithelium exhibiting reactive changes. Most commonly, AP arise in the middle ear cleft in response to a longstanding local inflammatory process such as chronic otitis media (Table 5). The otologic tissue specimen submitted as ‘polyp’ should be evaluated with the knowledge of the spectrum of diseases known to clinically mimic aural polyp disease (Table 6). The significance of histologically confirmed AP is the association with active middle ear disease and the possibility of cholesteatoma [54]. The frequency of finding AP and cholesteatoma varies from 25 to 60%, and is important because the presence of cholesteatoma requires surgical intervention [55–57]. Although some have suggested that histologic examination of AP material can predict the presence of underlying cholesteatoma [58], others investigators view this assertion with caution due to the possibility of a high false positive rate [59], particularly where a clinic-based sampling method of AP is used. Keratinous debris or fragmented, superficial and inflamed samples of the medial aspect of the external canal lining has the potential to overlap with histologic features of cholesteatoma.
Table 6.
Entities clinically characterized as aural polyp in external and/or middle ear
| Infectious/inflammatory |
| Granulomatous inflammation |
| Acute localized otitis externa (furunculosis) |
| Tuberculous otitis |
| Otomycosis |
| Primary tumors/pseudotumors and secondary neoplasms |
| Middle ear adenoma |
| Neural tumors |
| Dermal nevus |
| Meningioma |
| External auditory canal osteoma |
| Rhabdomyosarcoma |
| Squamous cell carcinoma |
| Langerhan’s cell histiocytosis |
| Metastatic disease: renal cell carcinoma, melanoma |
| Malignant salivary gland tumor (primary or secondary extension from parotid gland) |
| Extension of synovial or joint pathology from temporomandibular joint region |
No definite age or gender predilection is noted. Patients present with otorrhea, conductive hearing loss, and depending on the location and size of the polyp a ‘mass-like’ sensation. Otalgia is uncommonly reported. On examination, a friable polypoid mass is identified and can originate from any part of the mucosa in the middle ear cleft or mastoid cavity [24]. External canal polyps are described, but most often these represent middle ear aural polyps extending into the canal through an existing a TM defect [60]. The majority of inflammatory polyps associated with chronic otitis media (with or without cholesteatoma) are unilateral [54]. Bilateral AP are uncommon, and most often occur in patients with EOM and aspirin exacerbated respiratory disease (AERD) [61–64]. Rarely, bilateral aural polyps have been reported in association with HIV [65].
Histopathologic Findings
Depending on activity of underlying disease, AP may consist of edematous, hypercellular granulation tissue rich in capillary sized vasculature or a less cellular, more fibrous core with a decreased vascular component. The tissue may or may not have associated lining epithelium (Fig. 10a, b). When middle ear epithelium is present it can appear as cuboidal, or a reactive pseudostratified columnar epithelium with cilia, goblet cells or glandular metaplasia. The presence of keratinizing stratified squamous epithelium within a middle ear tissue biopsy may be reported as such with clinical correlation essential to ascertain the presence of cholesteatoma, similar to chronic suppurative OM [61]. Scattered eosinophils may be seen, but dense eosinophilia is regarded as unusual in inflammatory and may represent eosinophilic aural polyps [61]. Eosinophilic aural polyp is a non-neoplastic polypoid fibrogranulation tissue aggregate with pools of stromal mucin and numerous eosinophils that more closely resembles a sample expected from the sinonasal tract than from the middle ear cleft and occurs in the setting of eosinophilic otitis media (see Otitis Media) [61].
Fig. 10.
Aural (otic) polyp. a Aural polyp (H&E, 30x). b Granulation tissue with exuberant inflammation (H&E, 300x)
Treatment
An aural polyp that clinically persists despite conservative treatment should be removed for histological examination [59]. Exploration in the operating theater allows for an adequate view of the TM, meticulous assessment for the presence of cholesteatoma, extent of middle ear disease, and avoids blind removal of polyp tissue that may be firmly adherent to important bony or neural anatomy [56]. Intraoperative consultation with pathology may be requested to evaluate unusual clinical findings with the potential to impact intraoperative decision making [59, 66, 67]. When clinical, radiographic and/or histologic evidence confirms the presence of cholesteatoma, standard treatment is surgery as there is no predictable medical therapy known for the treatment of cholesteatoma.
Foreign Body and Foreign Body Reaction
Foreign Body
Background
Inanimate foreign bodies are the most common type of intraural foreign body (FB), but a wide range of animate foreign bodies are also encountered [68, 69]. Although diagnosis of intraural FB is primarily a clinical diagnosis, there are some considerations that may be of interest to the practicing pathologist. For this reason, a selected review of the general medical importance of ticks and mites and fly larvae (‘maggots’), clinical features, specimen handling and reporting is presented herein.
The medical significance of tissue infestation by ticks, mites and fly larvae is related to their role as biologic vectors of disease, mechanical vectors of disease and the locoregional tissue pathology that results as a consequence of parasitic behavior [70]. Ticks, particularly female ticks, are known as biologic vectors for diseases such as Rocky Mountain Spotted Fever, and Tularemia among others [70]. Fly larvae can act as passive carriers (mechanical vectors) of bacteria, giving rise to complications such as bacterial superinfection and tetanus [70, 71]. Local or locoregional complications of arthropod attachment TM or canal skin can occur as otalgia, bleeding, or local skin or tympanic membrane wounds. Rarely, facial paralysis or an acute ascending flaccid paralysis (‘tick paralysis’) can occur [68, 72].
Otoacariasis
The term otoacariasis refers to the presence of ticks or mites within the external auditory canal [73, 74]. Almost all ticks belong to one of two large families, Ixodidae (hard ticks, with a hard sclerotized dorsal plate) and Argasidae (soft ticks, with a leathery cuticle rather than a hard dorsal plate). Ticks from either of these families will bite human skin, but it is the hard ticks that are more likely to remain attached for long periods of time as they take a blood meal. The latter represents the period of greatest threat for disease transmission [70]. In the case of otoacariasis, the tick is clinically observed in the canal or on the TM and the diagnosis established. An improperly removed tick can result in a foreign body giant cell response to retained tick parts [68].
Mites such as Sarcoptes Scabiei (scabies) and follicle mites (Demodex spp.) reside in the epidermis and folliculo-sebaceous units. Sarcoptes scabiei are the highly contagious agent of scabies, most commonly transferred by direct skin contact [70]. Scabies is accompanied by intense pruritus. The burrows in the skin appear clinically as short curved yellow-grey lines on the skin, commonly on the hands and feet, and skin scrapings are used to confirm the presence of mites. If skin scrapings fail to reveal scabies but the clinical suspicion persists, tissue sampling may show mites, eggs or scybala in the superficial layers of the epidermis. Coiled structures thought to represent the remnants of eggs (so called ‘pink pigtail’) within the stratum corneum are suggestive of scabies infestation (Fig. 11). Most times, the face is spared in scabies, but in children and immunocompromised patients the face or even the external ear canal may be involved and when untreated, scabies typically persists and spreads [75]. Demodex spp. mites are saprophytic mites in hair follicles and sebaceous glands. In most cases they are nonpathogenic, but the use of topical steroids, co-existing dermatologic disease, or density of mites may be associated with enhanced pathogenicity [76]. Mites within the ear canal can cause symptoms such as pruritus or clinical manifestations similar to chronic otitis externa [76, 77]. Mites have also been implicated in otophyma, and similar to nasal enlargement seen in rhinophyma, there is clinical enlargement of the external ear in otophyma, which can clinically resemble chronic otitis externa. Microscopically, otophyma is composed of dense fibrous tissue, perivascular and perifollicular lymphohistiocytic infiltrate, and granuloma formation. Demodex spp are found in the apopilosebaceous units [77, 78].
Fig. 11.
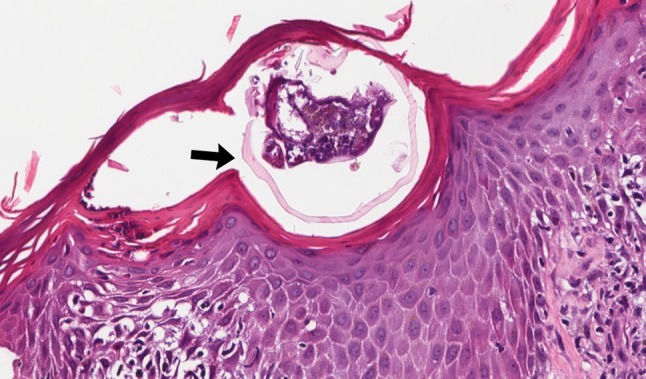
Scabies. Cross section of mite within parakeratotic, spongiotic epidermis (black arrow) (H&E, 200x)
Aural Myiasis
Some larval species are used for medical debridement, however the focus of the current review is to examine myiasis as it relates to non-therapeutic infestation of live host tissue, human or animal, by dipterous (two-winged) fly larvae in the external canal and middle ear (aural myiasis or otomyiasis). Myiasis can be categorized ecologically using the relationship between the host and parasite (obligatory, facultative, accidental) or categorized by the affected site of tissue invasion. In obligatory myiasis, fly larvae depend on healthy host tissue for its development requirements which is in contrast to facultative myiasis, where feeding occurs on decaying or necrotic tissue. Accidental myiasis occurs when eggs or larvae are unintentionally ingested but survive this event and continue to proliferate. From a clinical standpoint, establishing the nature of the parasitic relationship may be difficult and clinical categorization using the site affected by myiasis is more useful and familiar. Cutaneous myiasis, particularly within existing wounds, is the most common presentation among other described sites such as enteric, ocular, nasopharyngeal, aural, oral and urogenital myiasis [70, 71].
Female flies are attracted to olfactory queues associated with wounds, decaying or necrotic tissue and settle in these tissues to deposit eggs. Risks for myiasis in general include one or more of the following: tropical geographic location, poor hygiene, low socioeconomic status, crowded living conditions, a debilitated state and/or presence of condition that limits the ability to protect oneself from flies settling on skin surfaces [70, 79]. Head and neck human wound myiasis is rarely reported as originating in the United States, and the presence of an underlying cutaneous malignancy represents the most common association [79]. Within subsites of the head and neck, aural myiasis is very rare [69, 80, 81]. Worldwide, the two most common types of larvae associated with aural myiasis are flesh flies (sarcophagidae) and blowflies (calliphoridae) [69]. Affected patients report otalgia, otorrhea, tinnitus/aural noise such as crackling, or the sensation of movement in the ear. When aural myiasis is identified clinically, the integrity of the TM and evaluation of adjacent structures is critical due to the possibility of tunneled channels that may compromise inner ear, brain, sinonasal passages/globe and vascular channels with the risk of massive hemorrhage [82, 83]. Treatment of aural myiasis is debridement of larvae and associated non-viable tissue (Fig. 12). Recurrence can occur but is uncommon [80]. Fly larvae can represent mechanical vectors of bacteria, therefore consideration of antibiotic therapy, tetanus prophylaxis or formal infectious disease consultation may be prudent [71, 79].
Fig. 12.

Fly larvae (Calliphoridae family) removed from middle ear cleft of elderly female with active chronic suppurative otitis media
Transport of Animate Foreign Body
To preserve macroscopic morphology of the arthropod, the extracted FB should be placed in 70–90% ethanol [70] or 70% isopropyl alcohol [71]. Formalin can be used but is less desirable it alters the properties of the FB, and may increase difficulty in processing [70, 71]. Classification of the arthropod may be more valuable to patient care than reporting of histologic findings, therefore when only a single arthropod is received (ex. single tick) in the pathology lab within other submitted tissues, it may be advisable to grossly identify the FB and record relationship, but submit the FB separately to the microbiology lab for classification (see Reporting, next) [70]. Mites and some cases with innumerable fly larvae may be largely inseparable from tissues and processed for routine histopathologic exam if microscopic documentation is also desired.
Reporting of Animate Foreign Body
Identifying and characterizing arthropods received in the lab can be challenging due to the diversity of the specimens, infrequency of request, and relative level of expertise in any one laboratory. Ticks and mites should be reported to the level of the genus, but species level if possible. Female ticks are more likely to take a blood meal and reporting the tick gender may be important. Evaluation of myiasis-causing larvae should at least include the classification of the larval family. Accurate reporting of the species of larvae may be difficult, and this, or identification of any other arthropod that falls outside the purview of the lab expertise could be referred to state or local health departments, university or veterinary programs with entomologists or parasitologists for examination and reporting. Identification of larvae engaged in obligatory myiasis may be important from a public health perspective [70].
Myospherulosis/Foreign Body Reaction
Background
Myospherulosis is a foreign body reaction first described in 1969 by McClatchie et al., and named for its spheruloid histologic features [84]. Although initially suspected to represent a fungal or other infection, myospherulosis has subsequently been confirmed to arise as a result of an interaction between erythrocytes and petrolatum, lanolin and other lipid-rich materials [85]. The year 1977 marked the first description of myospherulosis within sites of the head and neck (sinonasal and middle ear) [86]. Myospherulosis of the middle ear/mastoid occurs in patients with chronic ear disease and most often, a prior surgical procedure on the affected ear (Table 5). It is a clinically significant condition because it is preventable, is associated with symptoms such as otorrhea and otalgia, has space-occupying clinical or radiographic features that creates confusion with middle ear infectious or inflammatory processes, and the current recommended treatment is surgical excision [86, 87].
Histopathologic Findings
At low magnification, isolated and/or clustered small and large rounded pseudocystic spaces are identified within mild to moderately inflamed dense fibrous connective tissue. At higher magnification, many of the pseudocystic spaces are devoid of contents, while others contain scattered red-brown spherical bodies representing the altered red blood cells, along with scant debris. When the red blood cells (‘endobodies’, 5–7 µm) are closely packed within a pseudocyst (‘parent body’, up to 250 µm), the arrangement resembles a partly filled bag of marbles—a finding noted in the original description of myospherulosis [84, 88]. Foreign body giant cells with a compressed and flattened appearance line the edges of the pseudocysts, and a mild to moderate lymphoplasmacytic infiltrate present within fibrous connective tissue (Fig. 13a, b) [84, 87]. Histiocytes and lipogranulomatous inflammation may be identified [89]. Myospherulosis is negative for polarizable exogenous material but detection of small flecks of polarizable exogenous material representing prior surgical site changes in the adjacent fibrous connective tissue wall would not preclude the diagnosis. Special stains GMS and PAS are negative in the endobodies.
Fig. 13.
Myospherulosis. a Dilated pseudocysts separated by dense fibrous connective tissue (H&E, 100x). b Compressed giant cells surround pseudocysts containing altered erythrocytes (H&E, 200x)
Differential Diagnosis
Organisms such as rhinosporidiosis, coccidiomyosis and occasionally cryptococcus, enter the differential diagnosis due to their rounded, spheruloid appearance. Useful criteria to differentiate these infectious processes include assessment of the apparent size of the sporangium (enclosing parent body), and the internal endospores. The sporangium of rhinosporidium measure 250–300 µm, and the enclosed endospores of rhinosporidiosis can measure 6–10 µm, similar to myospherulosis, however GMS staining would highlight the organisms in rhinosporidiosis. Coccidiomycosis is smaller, with sporangia of 30–60 µm and endospores measuring 2–5 µm [86]. Though cryptococcus exhibits a sheet-like growth rather than sporangium/endospore arrangement, the small sized spherules within hemorrhagic tissue spaces can appear similar to myospherulosis (Fig. 14a), however similar to other true fungal forms, will demonstrate a positive reaction to GMS, PAS and/or mucicarmine special stains.
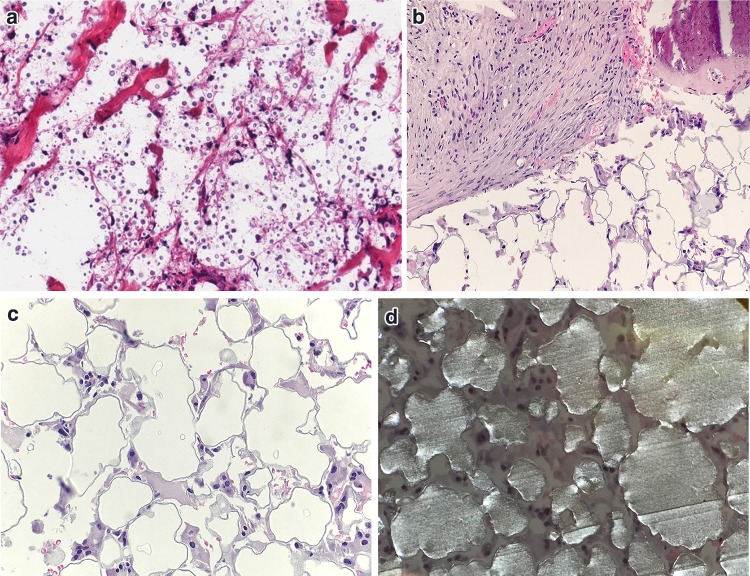
Fig. 14.
Differential Diagnosis of myospherulosis. a Degenerated tissue invaded by cryptococcus (H&E, 200x). b Tissue with failed Plastipore® ossicular implant removed from middle ear (H&E, 100x). c Higher power magnification of failed Plastipore® ossicular implant with pseudocystic spaces (H&E, 200x). d Plastipore® ossicular implant under polarized light exam (H&E, 200x)
A spectrum of autograft, homograft and synthetic materials have been utilized over the past several decades as components of tympanic membrane or ossicular grafts [90]. A fragment of failed synthetic graft material from the middle ear, particularly porous polyethylene (Plastipore®) or thermal fused high density polyethylene sponge (Polycel®) can mimic the multicystic spaces of myospherulosis. The histologic appearance of the material is related the average pore size at 250 µm, and associated inflammatory infiltrate including giant cells, however rarely internal endobody formation is seen [91]. Polarized light exam will further distinguish synthetic ossicular graft material as sheets of polarizable material (Fig. 14b–d). The presence of sheets of polarizable material is not a feature of myospherulosis.
Treatment
In the middle ear, myospherulosis is treated by removing the affected tissue and diseased mucosa under operating microscope guidance. Recurrence is rare [85, 89].
Fungal Diseases of the Ear
Introduction
Agents responsible for aural fungal infection include molds such as Aspergillus niger, Aspergillus fumigatus, Aspergillus flavus; and yeasts such as Candida albicans and Candida parapsilosis. In the skin lined elements and apopilosebaceous apparatus of the ear, Trichophyton spp. occur [92]. While considered rare, Candida auris is highlighted here as it was initially identified in the external auditory canal and represents an emerging public health threat. First identified in 2009, Candida auris was isolated from the discharge material in the ear canal of a hospitalized, elderly patient in Japan [93]. Since that time, C. auris has demonstrated the ability to cause outbreaks in healthcare settings, multidrug resistance and accurate identification remains a challenge. In the US between May 2013 and August 2016, C. auris was isolated from seven immunocompromised patients, one with known skull base osteomyelitis [94]. To assess and study the disease in this country, the Centers for Disease Control (CDC) issued a clinical alert in June 2016 with a request for formal reporting of cases of C. auris aimed to identify probable and confirmed cases in each state [94]. As of March 31, 2018 the CDC confirmed 257 cases of infection related to C. auris in the United States and documented over 475 patients known to be colonized with this pathogen https://www.cdc.gov/fungal/diseases/candidiasis/tracking-c-auris.html. The nature of the relationship between this organism and the ear and temporal bone disease awaits further study.
Types of Aural Fungal Infections
Otomycosis
Most commonly, the term otomycosis is used to refer to superficial (non-tissue invasive) mold or yeast infections present in keratinous debris within the EAC [92]. In this sense, otomycosis can occur under the same conditions that predispose to bacterial infection of the EAC, such as changes in the local canal moisture, alterations in the cerumen layer, and/or local trauma. Fungal otitis externa, synonymous with otomycosis, can evolve during or after antibiotic treatment of acute bacterial otitis externa due to selective pressures on the microbial environment, or recent use of topical steroid in the external ear.
Although the term otomycosis usually refers to non-invasive fungus detected within the external canal, it can be applied to characterize the histologic finding of non-invasive fungal forms within middle ear, and in this setting it is most frequently identified in the keratinous debris of middle ear cholesteatoma [95, 96]. In patients with chronic suppurative OM (with or without cholesteatoma) the presence of persistent or recurrent otorrhea macerates the canal epithelium, optimizing local conditions for fungal growth within the ear canal. The defect in the TM allows fungal elements to enter into the middle ear tissues including keratin debris of cholesteatoma [92, 95]. The significance of mucoinflammatory debris (appearing microscopically similar to eosinophilic otitis media) within the middle ear/mastoid associated with fungal hyphae is not well described, but appears to occur in patients with long-standing chronic ear disease and may represent a form of hypersensitivity to the fungal elements in otomycosis [37, 38].
The primary symptom of classic external canal otomycosis is pruritus but otorrhea may also be reported. On examination, the skin of the canal is edematous, erythematous, and commonly reveals thickened white or grey debris which may have dark punctate flecks within the material along the canal [92]. The clinical diagnosis of classic otomycosis can be straightforward, requiring only clinical history and recognition of fungal debris present within the EAC on otoscopic examination. Other cases may more closely mimic bacterial infection or dermatologic conditions and thus be more problematic to accurately identify by clinical exam alone. In these situations, tissue samples may be sent for histopathologic examination to search for fungal elements in the stratum corneum and/or samples of tissue, debris or secretions in the canal can be submitted for culture [92, 97].
Histopathologic Findings
The routine processing of tissue specimens from the canal, TM, or middle ear/mastoid cholesteatoma can be evaluated for the presence of fungal forms in the stratum corneum, keratinous debris of follicular structures or within the cholesteatoma. In full thickness sections of external canal epithelium, the tissues may exhibit acute and chronic inflammation in response to fungal overgrowth. Special stains such as GMS or PAS are helpful in highlighting fungal elements. There should be no infiltration of fungal forms into supporting tissues such as stroma, bone, cartilage, nerve or blood vessels [92]. Occasionally, the matted debris is curetted from the external auditory canal and submitted for microscopic examination. Tangled masses of fungal hyphae, bacteria, inflammation and scales of keratinous debris support the diagnosis of otomycosis (Fig. 15 a,b).
Fig. 15.
Otomycosis of external canal in healthy patient with intact TM. a Aggregate of keratinous debris, bacteria, inflammation and fungal hyphae (H&E, 50x). b Higher power magnification of external canal keratinous debris, bacterial and fungal forms (H&E, 200x). Grocott Gomori special stain highlights fungal hyphae (H&E, 200x)
Treatment
Superficial fungal infections/chronic colonization of the external ear is managed with debridement of the fluffy white-to-dark material, topical antifungal treatment and management of any contributing factors if present [97]. In the presence of a TM defect, the ototoxic potential of any antifungal agent must also be considered. The significance of otomycosis has long been controversial since it most often represents an innocuous event, easily treated once recognized.
Acute Tissue Invasive Fungal Infection
Although an uncommon occurrence, an acute tissue invasive fungal infection can involve the ear and surrounding tissues particularly in an immunocompromised host. Immune compromise may be related to any number of disorders such as hematolymphoid malignancy, transplantation, diabetes or HIV. Aspergillus is most frequently identified, while Mucorales genera is less common in this site [98, 99]. Clinically, an ulcerated lesion on the skin of the tragus, periauricular skin or a site of injury is present and malignancy, viral lesion or fungal infectious processes are high on the clinical differential diagnosis. The diagnosis must be confirmed by tissue biopsy where histological features include identification of fungal elements within deep tissue planes, tissue necrosis, ulceration, and the possibility of angioinvasion. Treatment of an acute tissue invasive fungal infection consists of debridement, and systemic antifungal therapy. Material for fungal culture may be helpful in selecting appropriate fungal therapy in this group of patients, particularly those who may have limited renal or hepatic reserve due to comorbid conditions.
Fungal Necrotizing Otitis Externa
Clinical features of fungal necrotizing otitis externa (F-NOE) are similar to NOE caused by bacteria, though F-NOE may represent a more aggressive form of NOE [100] with higher rates of seventh cranial nerve damage, and reduced 5 year survival compared to non-fungal NOE [101]. It has been suggested that the presence of fungal pathogens and/or pathogenicity of some including Aspergillus sp. is enhanced by disrupting bacterial flora in the course of recent or concurrent antibiotic treatment related to NOE [101, 102]. F-NOE, in contrast to the acute invasive fungal infection of otologic tissues described in the preceding section, may represent more of a chronic form of tissue invasive disease. With overlapping clinical features and culture negative NOE, the question remains how to best identify a patient affected with F-NOE. A high index of suspicion is required and F-NOE, or shift from bacterial related NOE to F-NOE may be suspected in cases where clinical response lags or begins to plateau with worsening of clinical symptoms worsen despite adequate antibiotic therapy. Deep tissue biopsies can be helpful for in providing repeat culture material and to evaluate tissue for the presence of fungal elements on tissue sections. In the face of clinically refractory disease with negative cultures but a persistent clinical suspicion of F-NOE, material for ELISA or PCR assay has been reported as successful in fungal detection and identification [4, 92].
Conclusion
In conclusion, the present review of non-neoplastic conditions affecting the external and middle ear tissues highlights not only the overlapping features, but where possible, the unique clinical and histologic features are identified to facilitate interdisciplinary communication and care of the patient with otologic disease.
Compliance with Ethical Standards
Conflict of interest
The authors individually declare no conflicts of interest.
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Bojrab DI, Bruderly T, Abdulrazzak Y. Otitis externa. Otolaryngol Clin North Am. 1996;29:761–782. [PubMed] [Google Scholar]
- 2.Kesser BW. Assessment and management of chronic otitis externa. Curr Opin Otolaryngol Head Neck Surg. 2011;19:341–347. doi: 10.1097/MOO.0b013e328349a125. [DOI] [PubMed] [Google Scholar]
- 3.Wipperman J. Otitis externa. Prim Care. 2014;41:1–9. doi: 10.1016/j.pop.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Gruber M, Roitman A, Doweck I, et al. Clinical utility of a polymerase chain reaction assay in culture-negative necrotizing otitis externa. Otol Neurotol. 2015;36:733–736. doi: 10.1097/MAO.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer PE, Kelemen G. Pyocyaneous osteomyelitis of the temporal bone, mandible and zygoma. Laryngoscope. 1959;69:1300–1316. doi: 10.1288/00005537-195910000-00006. [DOI] [Google Scholar]
- 6.Chandler JR. Malignant external otitis. Laryngoscope. 1968;78:1257–1294. doi: 10.1288/00005537-196808000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Evans IT, Richards SH. Malignant (necrotising) otitis externa. J Laryngol Otol. 1973;87:13–20. doi: 10.1017/S0022215100076568. [DOI] [PubMed] [Google Scholar]
- 8.Chawdhary G, Liow N, Democratis J, Whiteside O. Necrotising (malignant) otitis externa in the UK: a growing problem. Review of five cases and analysis of national Hospital Episode Statistics trends. J Laryngol Otol. 2015;129:600–603. doi: 10.1017/S002221511500105X. [DOI] [PubMed] [Google Scholar]
- 9.Goh JPN, Karandikar A, Loke SC, Tan TY. Skull base osteomyelitis secondary to malignant otitis externa mimicking advanced nasopharyngeal cancer: MR imaging features at initial presentation. Am J Otolaryngol. 2017;38:466–471. doi: 10.1016/j.amjoto.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Glikson E, Sagiv D, Wolf M, Shapira Y. Necrotizing otitis externa: diagnosis, treatment, and outcome in a case series. Diagn Microbiol Infect Dis. 2017;87:74–78. doi: 10.1016/j.diagmicrobio.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Franco-Vidal V, Blanchet H, Bebear C, Dutronc H, Darrouzet V. Necrotizing external otitis: a report of 46 cases. Otol Neurotol. 2007;28:771–773. doi: 10.1097/MAO.0b013e31805153bd. [DOI] [PubMed] [Google Scholar]
- 12.Stern Shavit S, Soudry E, Hamzany Y, Nageris B. Malignant external otitis: factors predicting patient outcomes. Am J Otolaryngol. 2016;37:425–430. doi: 10.1016/j.amjoto.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Narozny W, Kuczkowski J, Mikaszewski B. Infectious skull base osteomyelitis–still a life-threatening disease. Otol Neurotol. 2006;27:1047–1048. doi: 10.1097/01.mao.0000235372.39998.7c. [DOI] [PubMed] [Google Scholar]
- 14.Chen CN, Chen YS, Yeh TH, Hsu CJ, Tseng FY. Outcomes of malignant external otitis: survival vs mortality. Acta Otolaryngol. 2010;130:89–94. doi: 10.3109/00016480902971247. [DOI] [PubMed] [Google Scholar]
- 15.Ostfeld E, Segal M, Czernobilsky B. Malignant external otitis: early histopathologic changes and pathogenic mechanism. Laryngoscope. 1981;91:965–970. doi: 10.1288/00005537-198106000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Bernheim J, Sade J. Histopathology of the soft parts in 50 patients with malignant external otitis. J Laryngol Otol. 1989;103:366–368. doi: 10.1017/S0022215100108977. [DOI] [PubMed] [Google Scholar]
- 17.Mattucci KF, Setzen M, Galantich P. Necrotizing otitis externa occurring concurrently with epidermoid carcinoma. Laryngoscope. 1986;96:264–266. doi: 10.1288/00005537-198603000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Schilder AG, Chonmaitree T, Cripps AW, et al. Otitis media. Nat Rev Dis Primers. 2016;2:16063. doi: 10.1038/nrdp.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van der Poel NA, van Spronsen E, Dietz de Loos DA, Ebbens FA. Early signs and symptoms of intracranial complications of otitis media in pediatric and adult patients: a different presentation? Int J Pediatr Otorhinolaryngol. 2017;102:56–60. doi: 10.1016/j.ijporl.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 20.Bhutta MF, Thornton RB, Kirkham LS, Kerschner JE, Cheeseman MT. Understanding the aetiology and resolution of chronic otitis media from animal and human studies. Dis Model Mech. 2017;10:1289–1300. doi: 10.1242/dmm.029983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schilder AG, Marom T, Bhutta MF, et al. Panel 7: otitis media: treatment and complications. Otolaryngol Head Neck Surg. 2017;156:88–105. doi: 10.1177/0194599816633697. [DOI] [PubMed] [Google Scholar]
- 22.Ferlito A. Pathology of chronic suppurative otitis media. A histological and histochemical study. ORL J Otorhinolaryngol Relat Spec. 1974;36:257–274. doi: 10.1159/000275182. [DOI] [PubMed] [Google Scholar]
- 23.Salvinelli F, Trivelli M, Greco F, Linthicum FH., Jr Chronic otitis media: histopathological changes: a post mortem study on temporal bones. Eur Rev Med Pharmacol Sci. 1999;3:175–178. [PubMed] [Google Scholar]
- 24.Youngs R. The histopathology of mastoidectomy cavities, with particular reference to persistent disease leading to chronic otorrhoea. Clin Otolaryngol Allied Sci. 1992;17:505–510. doi: 10.1111/j.1365-2273.1992.tb01706.x. [DOI] [PubMed] [Google Scholar]
- 25.Kamimura M, Balaban CD, Sando I, Ganbo T, Suzuki C. Cellular distribution of mucosa-associated lymphoid tissue with otitis media in children. Ann Otol Rhinol Laryngol. 2000;109:467–472. doi: 10.1177/000348940010900505. [DOI] [PubMed] [Google Scholar]
- 26.Michal M, Skalova A, Kazakov DVet. Mixed epithelial and stromal tumor of the middle ear: the first case report. Hum Pathol. 2017;61:199–204. doi: 10.1016/j.humpath.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Azadeh B, Dabiri S, Moshfegh I. Malakoplakia of the middle ear. Histopathology. 1991;19:276–278. doi: 10.1111/j.1365-2559.1991.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 28.Tomioka S, Kobayashi T, Takasaka T. Intractable otitis media in patients with bronchial asthma (eosinophilic otitis media) In: Sanna M, editor. Cholestoma and mastoid surgery. Rome: CIC Edizioni Internazionali; 1997. p. 8513. [Google Scholar]
- 29.Iino Y, Tomioka-Matsutani S, Matsubara A, Nakagawa T, Nonaka M. Diagnostic criteria of eosinophilic otitis media, a newly recognized middle ear disease. Auris Nasus Larynx. 2011;38:456–461. doi: 10.1016/j.anl.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Kanazawa H, Yoshida N, Iino Y. New insights into eosinophilic otitis media. Curr Allergy Asthma Rep. 2015;15:76. doi: 10.1007/s11882-015-0577-2. [DOI] [PubMed] [Google Scholar]
- 31.Childers AL, Gruen J, Sayeed S, Powers CN, Coelho DH. Eosinophilic otitis media. Otol Neurotol. 2014;35:e206–e207. doi: 10.1097/MAO.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 32.Yang B, Brook CD. The role of allergy in otologic disease. Otolaryngol Clin North Am. 2017;50:1091–1101. doi: 10.1016/j.otc.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Saliba I, Alzahrani M, Weng X, Bestavros A. Eosinophilic otitis media diagnosis using flow cytometric immunophenotyping. Acta Otolaryngol. 2018;138:110–115. doi: 10.1080/00016489.2017.1385845. [DOI] [PubMed] [Google Scholar]
- 34.Blake DM, Vazquez A, Tomovic S, Jyung RW. Otologic manifestation of Samter triad. Ear Nose Throat J. 2014;93:238–240. [PubMed] [Google Scholar]
- 35.Nishizawa H, Matsubara A, Nakagawa T, et al. The role of periostin in eosinophilic otitis media. Acta Otolaryngol. 2012;132:838–844. doi: 10.3109/00016489.2012.668708. [DOI] [PubMed] [Google Scholar]
- 36.Nader ME, Bell D, Ginsberg L, DeMonte F, Gunn GB, Gidley PW. The first reported case of primary intestinal-type adenocarcinoma of the middle ear and review of the literature. Otol Neurotol. 2017;38:e364–e368. doi: 10.1097/MAO.0000000000001541. [DOI] [PubMed] [Google Scholar]
- 37.Zhu BZ, Bishop JA, Chien WW. A case of allergic fungal otomastoiditis with aural polyps. Otol Case Rep. 2017;2:4–6. [Google Scholar]
- 38.Chen CM, Chiang CW. Allergic fungal otomastoiditis: a case report. Laryngoscope. 2013;123:1040–1042. doi: 10.1002/lary.23582. [DOI] [PubMed] [Google Scholar]
- 39.Mills R, Hathorn I. Aetiology and pathology of otitis media with effusion in adult life. J Laryngol Otol. 2016;130:418–424. doi: 10.1017/S0022215116000943. [DOI] [PubMed] [Google Scholar]
- 40.Azadarmaki R, Westra W, Prasad S. Eosinophilic mucin otomastoiditis and otopolyposis: a progressive form of eosinophilic otitis media. Ann Otol Rhinol Laryngol. 2015;124:752–756. doi: 10.1177/0003489415577988. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson BJ, Seethala R, Wood WA. Eosinophilic bacterial chronic rhinosinusitis. Laryngoscope. 2007;117:2036–2040. doi: 10.1097/MLG.0b013e318123f2d7. [DOI] [PubMed] [Google Scholar]
- 42.Okada M, Suemori K, Takagi D, Teraoka M, Yamada H, Hato N. Comparison of localized and systemic otitis media with ANCA-associated vasculitis. Otol Neurotol. 2017;38:e50610. doi: 10.1097/MAO.0000000000001563. [DOI] [PubMed] [Google Scholar]
- 43.Harabuchi Y, Kishibe K, Tateyama K. Clinical features and treatment outcomes of otitis media with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (OMAAV): a retrospective analysis of 235 patients from a nationwide survey in Japan. Mod Rheumatol. 2017;27:87–94. doi: 10.1080/14397595.2016.1177926. [DOI] [PubMed] [Google Scholar]
- 44.Hisata Y, Sasaki E, Ishimaru K. Do you know otitis media with ANCA-associated vasculitis (OMAAV)? J Gen Fam Med. 2017;18:428–431. doi: 10.1002/jgf2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kukushev G, Kalinova D, Sheytanov I, Rashkov R. Two clinical cases of granulomatosis with polyangiitis with isolated otitis media and mastoiditis. Reumatologia. 2017;55:256–260. doi: 10.5114/reum.2017.71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akyigit A, Yalcin S, Etem EO, et al. Genetic polymorphisms affecting antioxidant enzymes are present in tympanosclerosis patients. J Laryngol Otol. 2016;130:928–933. doi: 10.1017/S0022215116008732. [DOI] [PubMed] [Google Scholar]
- 47.Tukaj C, Kuczkowski J, Sakowicz-Burkiewicz M, et al. Morphological alterations in the tympanic membrane affected by tympanosclerosis: ultrastructural study. Ultrastruct Pathol. 2014;38:69–73. doi: 10.3109/01913123.2013.833563. [DOI] [PubMed] [Google Scholar]
- 48.Ferlito A. Histopathogenesis of tympanosclerosis. J Laryngol Otol. 1979;93:25–37. doi: 10.1017/S0022215100086680. [DOI] [PubMed] [Google Scholar]
- 49.Branco C, Paco J. Does calcemia influence the onset of myringosclerosis after myringotomy with the insertion of ventilation tubes? Acta Otorrinolaringol Esp. 2017;68:3237. doi: 10.1016/j.otorri.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Bhaya MH, Schachern PA, Morizono T, Paparella MM. Pathogenesis of tympanosclerosis. Otolaryngol Head Neck Surg. 1993;109:413–420. doi: 10.1177/019459989310900305. [DOI] [PubMed] [Google Scholar]
- 51.Russell JD, Giles JJ. Tympanosclerosis in the rat tympanic membrane: an experimental study. Laryngoscope. 2002;112:1663–1666. doi: 10.1097/00005537-200209000-00025. [DOI] [PubMed] [Google Scholar]
- 52.Makishima K, Toriya Y, Inoue S, Nakashima T, Igarashi Y. Clinicopathologic studies in tympanosclerosis. Am J Otol. 1982;3:260–265. [PubMed] [Google Scholar]
- 53.McCadden L, Leonard CG, Primrose WJ. Bisphosphonate-induced osteonecrosis of the ear canal: our experience and a review of the literature. J Laryngol Otol. 2018;132:3724. doi: 10.1017/S0022215118000324. [DOI] [PubMed] [Google Scholar]
- 54.Shew M, Bush J, Tawfik O, Ator G. Middle ear aural polyp mimicking glomus tympanicum in a male adolescent. Otol Neurotol. 2017;38:e2113. doi: 10.1097/MAO.0000000000001470. [DOI] [PubMed] [Google Scholar]
- 55.Gliklich RE, Cunningham MJ, Eavey RD. The cause of aural polyps in children. Arch Otolaryngol Head Neck Surg. 1993;119:669–671. doi: 10.1001/archotol.1993.01880180089016. [DOI] [PubMed] [Google Scholar]
- 56.Hussain SS. Histology of aural polyp as a predictor of middle ear disease activity. J Laryngol Otol. 1991;105:268–269. doi: 10.1017/S0022215100115579. [DOI] [PubMed] [Google Scholar]
- 57.Veitch D, Brockbank M, Whittet H. Aural polyps and cholesteatoma. Clin Otolaryngol Allied Sci. 1988;13:395–397. doi: 10.1111/j.1365-2273.1988.tb00772.x. [DOI] [PubMed] [Google Scholar]
- 58.Milroy CM, Slack RW, Maw AR, Bradfield JW. Aural polyps as predictors of underlying cholesteatoma. J Clin Pathol. 1989;42:460–465. doi: 10.1136/jcp.42.5.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tay HL, Hussain SS. The management of aural polyps. J Laryngol Otol. 1997;111:212–214. doi: 10.1017/S002221510013693X. [DOI] [PubMed] [Google Scholar]
- 60.Spielmann PM, McKean S, White RD, Hussain SS. Surgical management of external auditory canal lesions. J Laryngol Otol. 2013;127:246–251. doi: 10.1017/S0022215112003155. [DOI] [PubMed] [Google Scholar]
- 61.Shen J, Peterson M, Mafee M, Nguyen QT. Aural polyps in Samter’s triad: case report and literature review. Otol Neurotol. 2012;33:774–778. doi: 10.1097/MAO.0b013e318259522f. [DOI] [PubMed] [Google Scholar]
- 62.Brobst R, Suss N, Joe S, Redleaf S. Bilateral inflammatory aural polyps: a manifestation of Samter’s triad. Int J Otolaryngol 2009:464958. [DOI] [PMC free article] [PubMed]
- 63.Lara-Sanchez H, Vallejo LA. Eosinophilic otitis media. N Engl J Med. 2017;376:e10. doi: 10.1056/NEJMicm1510852. [DOI] [PubMed] [Google Scholar]
- 64.Kumar MS, Panella NJ, Magliocca KR, Vivas EX. Otopolyposis with middle ear allergic mucin in a patient with allergic fungal rhinosinusitis. Ann Otol Rhinol Laryngol. 2016;125:862–865. doi: 10.1177/0003489416660112. [DOI] [PubMed] [Google Scholar]
- 65.Praveen CV, Terry RM, Elmahallawy M, Horsfield C. Pneumocystis carinii infection in bilateral aural polyps in a human immunodeficiency virus-positive patient. J Laryngol Otol. 2002;116:288–290. doi: 10.1258/0022215021910555. [DOI] [PubMed] [Google Scholar]
- 66.Xenellis J, Mountricha A, Maragoudakis P. A histological examination in the cases of initial diagnosis as chronic otitis media with a polypoid mass in the external ear canal. Auris Nasus Larynx. 2011;38:325–328. doi: 10.1016/j.anl.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Williams RA, Jackler RK, Corrales CE. Benign temporomandibular joint lesions presenting as masses in the external auditory canal. Otol Neurotol. 2017;38:563–571. doi: 10.1097/MAO.0000000000001354. [DOI] [PubMed] [Google Scholar]
- 68.Indudharan R, Ahamad M, Ho TM, Salim R, Htun YN. Human otoacariasis. Ann Trop Med Parasitol. 1999;93:163–167. doi: 10.1080/00034983.1999.11813406. [DOI] [PubMed] [Google Scholar]
- 69.Jervis-Bardy J, Fitzpatrick N, Masood A, Crossland G, Patel H. Myiasis of the ear: a review with entomological aspects for the otolaryngologist. Ann Otol Rhinol Laryngol. 2015;124:345–350. doi: 10.1177/0003489414557021. [DOI] [PubMed] [Google Scholar]
- 70.Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbiol Rev. 2014;27:48–67. doi: 10.1128/CMR.00008-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907–926. doi: 10.1016/j.jaad.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 72.Dana AN. Diagnosis and treatment of tick infestation and tick-borne diseases with cutaneous manifestations. Dermatol Ther. 2009;22:293–326. doi: 10.1111/j.1529-8019.2009.01244.x. [DOI] [PubMed] [Google Scholar]
- 73.Ariyarathne S, Apanaskevich DA, Amarasinghe PH, Rajakaruna RS. Diversity and distribution of tick species (Acari: Ixodidae) associated with human otoacariasis and socio-ecological risk factors of tick infestations in Sri Lanka. Exp Appl Acarol. 2016;70:99–123. doi: 10.1007/s10493-016-0056-z. [DOI] [PubMed] [Google Scholar]
- 74.Gokdogan O, Cakabay T, Baran H, Karabulut B, Tasdemir C, Vatansever Z. Otoacariasis: demographic and clinical outcomes of patients with ticks in the ear canal. Braz J Otorhinolaryngol. 2016;82:416–421. doi: 10.1016/j.bjorl.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsuo R, Honma M, Komatsu S, Minami-Hori M, Ishida-Yamamoto A, Iizuka H. External ear canal lesions of crusted scabies: a pitfall of recurrence? J Dermatol. 2015;42:1023–1024. doi: 10.1111/1346-8138.13021. [DOI] [PubMed] [Google Scholar]
- 76.Cevik C, Kaya OA, Akbay E, et al. Investigation of demodex species frequency in patients with a persistent itchy ear canal treated with a local steroid. J Laryngol Otol. 2014;128:698–701. doi: 10.1017/S0022215114001510. [DOI] [PubMed] [Google Scholar]
- 77.Klemm E, Haroske G, Wollina U. Otitis externa and myringitis due to demodicidosis. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:73–76. [PubMed] [Google Scholar]
- 78.Kahn SL, Podjasek JO, Dimitropoulos VA, Brown CW., Jr Excisional debulking and electrosurgery of otophyma and rhinophyma. Dermatol Surg. 2016;42:137–139. doi: 10.1097/DSS.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 79.Villwock JA, Harris TM. Head and neck myiasis, cutaneous malignancy, and infection: a case series and review of the literature. J Emerg Med. 2014;47:e37–e41. doi: 10.1016/j.jemermed.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 80.Hatten K, Gulleth Y, Meyer T, Eisenman DJ. Myiasis of the external and middle ear. Ann Otol Rhinol Laryngol. 2010;119:436–438. doi: 10.1177/000348941011900702. [DOI] [PubMed] [Google Scholar]
- 81.Sharan R, Isser DK. Aural myiasis. J Laryngol Otol. 1978;92:705–708. doi: 10.1017/S0022215100085972. [DOI] [PubMed] [Google Scholar]
- 82.Magliulo G, Gagliardi M, D’Amico R. Human aural myiasis. Otolaryngol Head Neck Surg. 2000;122:777. doi: 10.1016/S0194-5998(00)70218-X. [DOI] [PubMed] [Google Scholar]
- 83.Yuca K, Caksen H, Sakin YFet. Aural myiasis in children and literature review. Tohoku J Exp Med. 2005;206:125–130. doi: 10.1620/tjem.206.125. [DOI] [PubMed] [Google Scholar]
- 84.McClatchie S, Warambo MW, Bremner AD. Myospherulosis: a previously unreported disease? Am J Clin Pathol. 1969;51:699–704. doi: 10.1093/ajcp/51.6.699. [DOI] [PubMed] [Google Scholar]
- 85.Phillip V, Becker K, Bajbouj M, Schmid RM. Myospherulosis. Ann Diagn Pathol. 2013;17:383–389. doi: 10.1016/j.anndiagpath.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 86.Kyriakos M. Myospherulosis of the paranasal sinuses, nose and middle ear. A possible iatrogenic disease. Am J Clin Pathol. 1977;67:118–130. doi: 10.1093/ajcp/67.2.118. [DOI] [PubMed] [Google Scholar]
- 87.Gillespie CA, Clark WB, Finkelstein E, Devaney K. Myospherulosis of the mastoid antrum: a case report. Auris Nasus Larynx. 1990;16:199–207. doi: 10.1016/S0385-8146(12)80127-1. [DOI] [PubMed] [Google Scholar]
- 88.Culviner WT, Leonard DW, Wilhelmsen CL, Bolger WE. Experimental myospherulosis of the paranasal sinuses: a histologic rabbit study. Am J Rhinol. 2000;14:131–137. doi: 10.2500/105065800781692877. [DOI] [PubMed] [Google Scholar]
- 89.Lin HW, Handzel O, Faquin WC, Gopen Q. Myospherulosis from antibiotic ointment in the postoperative mastoid space. Am J Otolaryngol. 2010;31:205–208. doi: 10.1016/j.amjoto.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 90.Bahmad F, Jr, Merchant SN. Histopathology of ossicular grafts and implants in chronic otitis media. Ann Otol Rhinol Laryngol. 2007;116:181–191. doi: 10.1177/000348940711600304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Makek M, Mattox DE, Schmid S, Fisch U. Histology of synthetic ossicular prostheses. Arch Otolaryngol Head Neck Surg. 1988;114:1127–1130. doi: 10.1001/archotol.1988.01860220061024. [DOI] [PubMed] [Google Scholar]
- 92.Vennewald I, Klemm E, Otomycosis Diagnosis and treatment. Clin Dermatol. 2010;28:202–211. doi: 10.1016/j.clindermatol.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 93.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 94.Vallabhaneni S, Kallen A, Tsay S. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013-August 2016. MMWR. 2016;65:1234–1237. doi: 10.15585/mmwr.mm6544e1. [DOI] [PubMed] [Google Scholar]
- 95.Vennewald I, Wollina U. Cutaneous infections due to opportunistic molds: uncommon presentations. Clin Dermatol. 2005;23:565–571. doi: 10.1016/j.clindermatol.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 96.Vennewald I, Schonlebe J, Klemm E. Mycological and histological investigations in humans with middle ear infections. Mycoses. 2003;46:12–18. doi: 10.1046/j.1439-0507.2003.00835.x. [DOI] [PubMed] [Google Scholar]
- 97.Song JE, Haberkamp TJ, Patel R, Redleaf MI. Fungal otitis externa as a cause of tympanic membrane perforation: a case series. Ear Nose Throat J. 2014;93:332–336. doi: 10.1177/014556131409300811. [DOI] [PubMed] [Google Scholar]
- 98.Menachof MR, Jackler RK. Otogenic skull base osteomyelitis caused by invasive fungal infection. Otolaryngol Head Neck Surg. 1990;102:285–289. doi: 10.1177/019459989010200315. [DOI] [PubMed] [Google Scholar]
- 99.Chen D, Lalwani AK, House JW, Choo D. Aspergillus mastoiditis in acquired immunodeficiency syndrome. Am J Otol. 1999;20:561–567. [PubMed] [Google Scholar]
- 100.Stevens SM, Lambert PR, Baker AB, Meyer TA. Malignant otitis externa: a novel stratification protocol for predicting treatment outcomes. Otol Neurotol. 2015;36:1492–1498. doi: 10.1097/MAO.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 101.Bowles PF, Perkins V, Schechter E. Fungal malignant otitis externa. BMJ Case Rep. 2017. [DOI] [PMC free article] [PubMed]
- 102.Ling SS, Sader C. Fungal malignant otitis externa treated with hyperbaric oxygen. Int J Infect Dis. 2008;12:550–552. doi: 10.1016/j.ijid.2008.03.003. [DOI] [PubMed] [Google Scholar]