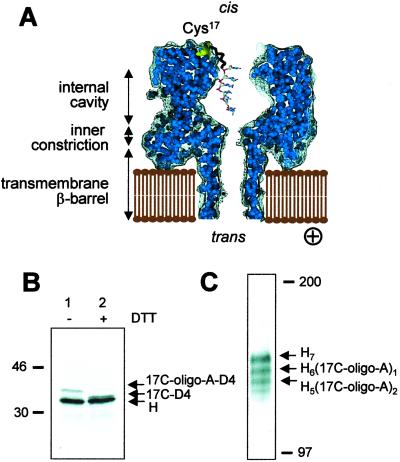
Figure 1.
Attachment of a single DNA oligonucleotide to the αHL pore. (A) A cross section of a model of a heteroheptameric αHL pore chemically modified with a single DNA oligonucleotide. The oligonucleotide is attached via a hexamethylene linker and a disulfide bond to cysteine residue 17 of a genetically engineered αHL subunit. ⊕ indicates the positive applied electrical potential that drives negatively charged molecules from the cis to the trans side of the bilayer. (B and C) Preparation of the αHL pore H6(17C-oligo-A)1. (B) Autoradiogram of an SDS–polyacrylamide gel after electrophoresis of a mixture of unmodified αHL monomers (H) and 17C-D4 monomers attached to oligo-A through a disulfide bond (17C-oligo-A-D4), in the absence (lane 1) and presence (lane 2) of the reducing agent DTT. (C) Autoradiogram of an SDS–polyacrylamide gel containing heteroheptamers formed by the assembly of a mixture of H and 17C-oligo-A-D4 monomers. Heptamers H7, H6(17C-oligo-A)1 and H5(17C-oligo-A)2 migrate in different gel bands because of an electrophoretic shift caused by the D4-tag in the 17C-oligo-A-D4 subunits. The positions of two molecular weight markers are indicated.