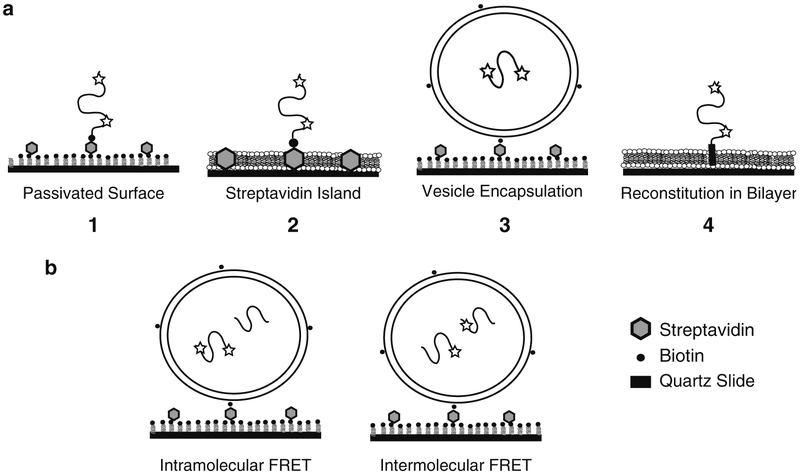
Fig. 2.
Experimental approaches to immobilized single protein molecules. (a) From left to right, (1) shows a schematic of a biotinylated single molecule directly immobilized on a passivated surface via streptavidin. Dyes are indicated by stars. The passivation can use bBSA or PEG (Subheadings 3.1.1 and 3.1.3). (2) Shows a protein immobilized on a streptavidin island surrounded by a deposited lipid bilayer (Subheading 3.1.2). (3) Shows a protein encapsulated within a lipid vesicle (Subheading 2.3.2), which is immobilized to a passivated surface (Subheading 3.2). (4) Shows a membrane protein reconstituted via the transmembrane domain into lipid bilayer (Subheading 2.3.3). A deposited bilayer is formed from vesicles containing reconstituted membrane proteins (Subheading 3.3). (b) Schematic showing co-encapsulation experiments to study protein binding. Intramolecular FRET (left) can monitor changes in a protein induced by binding to an unlabeled ligand protein. Intermolecular FRET (right) between two proteins, each singly labeled with donor or acceptor, can directly report on protein binding events.