Abstract
The adaptive response (AR) phenomenon generally describes a protective effect caused by a “priming” low dose (d AR) delivered after a period of time (Δt AR) before a higher “challenging” dose (D AR). The AR is currently observed in human cells if d AR, Δt AR, and D AR belong to (0.001-0.5 Gy), (2-24 hours), (0.1-5 Gy), respectively. In order to investigate the molecular mechanisms specific to AR in human cells, we have systematically reviewed the experimental AR protocols, the cellular models, and the biological endpoints used from the 1980s. The AR appears to be preferentially observed in radiosensitive cells and is strongly dependent on individual radiosensitivity. To date, the model of the nucleo-shuttling of the ATM protein provides a relevant mechanistic explanation of the AR molecular and cellular events. Indeed, the priming dose d AR may result in the diffusion of a significant amount of active ATM monomers in the nucleus. These ATM monomers, added to those induced directly by the challenging dose D AR, may increase the efficiency of the response to D AR by a better ATM-dependent DNA damage recognition. Such mechanistic model would also explain why AR is not observed in radioresistant or hyperradiosensitive cells. Further investigations at low dose are needed to consolidate our hypotheses.
Keywords: adaptive response, radiosensitivity, radiation, ATM
Introduction
A single dose of ionizing radiation (IR) triggers a cascade of physical, chemical, biological, and clinical events involving a large spectrum of spatiotemporal features (from femtoseconds to years; from femtometric to metric scale). Probably because the vaccination principles founded by Pasteur (note 1) were contemporary with the first descriptions of IR responses, the interpretation of the IR-induced phenomena was strongly influenced by immunology.1,2 This is notably the case of the hypothesis that the deleterious consequences of a single IR dose may be attenuated by a priming “stimulus” of IR. One of the most remarkable illustrations of this hypothesis is the notion of “radiovaccination” that Claudius Regaud proposed by 1914: He was convinced that “dose fractionation beyond ten days of radiotherapy led to induced radioresistance of the tumor.”3 However, the term “radiovaccination” was taken literally in a period that corresponded to a national campaign of vaccination against diphtheria and tuberculosis, and Regaud never identified the inherent immunological mechanisms that might be responsible for such induced resistance.3,4
The “immunological” hypothesis was also at the origin of a plethora of papers in which 2 different terms have been used to describe and interpret data: “adaptive response” (AR) and “hormesis.”5-7 Since the definition of these 2 terms have been so intricate through history, it is necessary here to define them in order to avoid any misinterpretation:
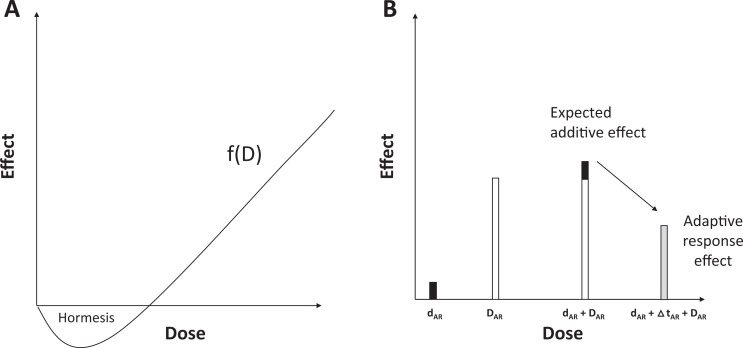
Adaptive response: A wording analysis via Google Ngram viewer revealed that “AR” is a very old term widely used in the 19th century in evolutionary biology, physiology, and zoology to evoke a long-term adaptation of an organism or a species for some years or some generations (Supplemental Figure S1). Progressively, the notion of time necessary to adaptation disappeared and the following definition of AR is now proposed: AR is “a process of adaptation which allows survival under adverse conditions.”8 With regard to the radiation research field, AR was first introduced in 1984 by Olivieri et al to describe a radiobiological phenomenon occurring after 2 successive doses9: the first one, the “priming” dose (d AR) precedes a certain period of time (Δt AR) and a higher “challenging” dose (D AR) under the following scheme: d AR + Δt AR + D AR with D AR > d AR. The AR phenomenon occurs when the effect of d AR + Δt AR + D AR is lower than that of D AR.9 The D AR systematically produces hazardous effects or is lethal. The priming dose d AR is generally interpreted as a stimulus of the cellular defenses to respond to the challenging dose D AR.7,10 However, the nature of such defenses is still unidentified (Figure 1).
Hormesis: The term “hormesis” was less frequently used than AR and was first introduced in 1943 (Supplemental Figure S1). When Southam and Ehrlich discovered that tree bark extracts stimulated fungi growing at low concentrations and was toxic at high concentrations, they proposed the term “hormesis” (from the ancient Greek, stimulus, rapid motion) to define “a stimulatory effect of subinhibitory concentrations of any toxic substance of any organism.”11 With regard to the radiation field, such a term was first introduced in 1980 by T.D. Luckey to describe a J- or U-shaped dose-dependent phenomenon, implying that hormesis is described by a continuous function of dose (or dose rate), with a specific threshold dose that separates an IR dose range in which stress exposure is positive and another one in which stress is detrimental12,13 (Figure 1). Strikingly, the notion of stimulatory effect was progressively replaced by the notion of beneficial effect, likely to be better opposed to the toxic effect observed at high doses. The nonlinear nature of the radiation hormesis phenomenon was an important aspect of the debate about the linear nonthreshold model.14,15
Hormesis versus AR: The abovementioned historical definition of “hormesis” may appear paradoxical, since hormesis means “stimulus,” which may suggest a first dose (the “stimulus”) that followed another one, similar to the abovementioned definition of AR. However, Southam and Ehrlich (1943) who first introduced this term used it to illustrate that doses belonging to a specific range may “stimulate” the cellular defenses. Furthermore, they did not apply a treatment consisting in repeated exposures to stress,11 and the hormetic J- or U-shaped dose-response curves reported by Luckey in the literature did not refer to a succession of doses but to a single dose or dose rate.5,12
Figure 1.
Differences between hormesis and adaptive response phenomena. A, Hormesis is defined as a continuous function of dose with which a stimulatory (beneficial?) effect occurs at subinhibitory doses. Hormesis is a single dose or dose-rate effect (it can be observed during a chronic exposure to radiation). B, Adaptive response (AR) is defined as an infra-additive effect observed after the succession of a priming d AR and a challenging dose D AR separated by a period of time Δt AR (the d AR + Δt AR + D AR scenario).
Hence, the abovementioned definitions, based on historical data and corresponding to specific experimental protocols, suggest that AR may lead to a reduction in risk linked to a high dose (“the adverse conditions”, the dose D AR) by another smaller one (d AR), while hormesis describes a dose range in which biological effects are beneficial without induction of another exposure. Hormesis and AR do not necessarily occur in the same dose range: As specified below, D AR is generally equivalent to some Gy, while hormesis is observed with much lower doses ranging from mGy to cGy.5 Since a reduction in the effect of some Gy is of interest in the radiodiagnosis and radiotherapy field, we focused here on the understanding of the AR phenomenon. The mechanisms specific to hormesis will be the subject of another report.
To date, while the individual response to IR is a major societal and public health issue, AR seems to depend on individual radiosensitivity, in terms of both occurrence and extent.16,17 In this review, in order to avoid any potential biases due to data obtained with other organisms, we have deliberately chosen to focus on the AR phenomenon observed in human cells in order to investigate the molecular and cellular mechanisms specific to AR. To this aim, we have systematically reviewed the experimental protocols, the cellular models and the biological endpoints. At the end of this review, we propose a mechanistic model explaining AR and its dependence vis-à-vis individual radiosensitivity.
Adaptive Response Throughout History: A Plethora of Different Protocols
We identified 3 historical periods in the research about AR:
1984 to 1989 period: Historically, the first report of a radiation-induced AR phenomenon in human cells was published in 1984 by Olivieri et al.9 The authors preincubated human lymphocytes with tritiated thymidine (0.1-0.01 μCi·mL−1) for 2 or 3 days and thereafter applied an X-ray dose of 1.5 Gy. In these conditions, the yield of chromatid aberrations was found to be lower than the sum of the yields of aberrations induced by tritiated thymidine and X-rays separately.9 It must be stressed that, in these conditions, the priming dose was delivered at a low-dose rate. Between 1984 and 1989, the same research group published some variants of this protocol notably with single doses (eg, d AR + Δt AR + D AR) and the following ranges: (0.01-0.05 Gy) for d AR, (16-32 hours) for Δt AR, and (0.25-1.5 Gy) for D AR.17-23 Sanderson and Morley23 and Sankaranarayanan et al24 reached the same conclusions with a similar irradiation protocol but with mutations frequency as biological endpoint. In 1989, Bosi and Olivieri raised for the first time the question of the individual response to AR. With the same experimental protocol as the one used in 1984, AR was analyzed in lymphocytes from 18 donors: About 10% donors did not show AR and about 20% elicited a synergistic effect.17 Hence, at the end of the 1980s, there was evidence that AR phenomenon depends on the individual status and occurs in human lymphocytes with chromatid aberrations or mutations frequency as endpoints, suggesting that AR may result in reducing genomic instability, cellular transformation and therefore cancer proneness.
1990 to 1999 period: In the 1990s, similar experimental protocols were applied but with larger d AR, Δt AR, and D AR ranges than those observed in the 1980s: (0.005-0.05 Gy) for d AR, (1-48 hours) for Δt AR, and (0.25-6 Gy) for D AR.16,25-35 Some new cellular models (eg, fibroblasts) and endpoints (eg, cell survival) were investigated. With regard to cell survival, Raaphorst and Boyden showed that AR strongly depends on the cell lines and also on Δt AR (but with d AR values between 0.5 and 2 Gy, ie, 10 times higher than the above range).16 With human fibroblasts and tumor cell lines, Seong et al confirmed the influence of Δt AR in the AR phenomenon: In their conditions, if Δt AR was less than 1 hour or greater than 30 hours, AR did not occur.28 Finally, this 1990 to 1999 period was particularly marked by an increasing number of reports investigating the individual response to AR. However, the genetic profile required for AR occurrence was not clearly defined.
2000 to 2007 period: From the 2000s, the considerable technological advances permitted to investigate AR-induced protein expression,36-38 DNA methylation,39 DNA damage repair and signaling assessed with various techniques,40-42 and cell death pathways.36,43-45 The d AR, Δt AR, and D AR ranges used in the reports published from 2000 were similar to those observed in the 1980s and the 1990s: (0.001-0.5 Gy) for d AR, (2-24 hours) for Δt AR, and (0.1-5 Gy) for D AR.36-51 All the endpoints tested in this period were linked to misrepaired DNA damage, mutations frequencies, and cellular transformation or to unrepaired DNA damage and cellular death. Hence, during this period, AR appeared to reduce either the risk of radiation-induced cancer or the cellular radiosensitivity. Let us remind that these 2 notions are not necessarily linked: For example, for a given cell line, some authors observed a significant AR response with mutations frequencies but not with clonogenic survival.51 It must also be stressed that some authors consider apoptosis as a protective and beneficial phenomenon as far as it could kill unstable cells specifically and reduce cancer risk.36 Care must be taken with such a hypothesis, since apoptosis is a very specific cell death pathway, strongly dependent on the p53 status and cell type.52 From the 2000s, the majority of AR reports investigated the individual response with an impressive variety of cell types: lymphocytes, lymphoblastoid cells, primary and transformed fibroblasts, keratinocytes, mammary epithelium, and tumor cells, suggesting that AR is not a phenomenon specific to a particular tissue (see the references cited above). Since all the AR-positive cellular models show various cell death pathways, apoptosis is not a specific feature of the AR phenomenon. To date, there is still no clear definition of individual, genetic, and cellular profile that would be required for an AR occurrence.
Summary: From the 1980s until to date, the AR response has been observed with the d AR + Δt AR + D AR scenario and with the following values: (0.001-0.5 Gy) for d AR delivered at dose-rate ranging, (1-48 hours) for Δt AR, and (0.1-6 Gy) for D AR. To the notable exception of the works from the group of Olivieri who used tritiated thymidine, in the great majority (>83%) of reports, d AR is delivered in less than 1 minute (ie, at dose rates ranging from 1 mGy.min−1 to 1 Gy.min−1). D AR is generally delivered in less than 5 minutes at about 1 Gy.min−1.
The Influence of the d AR, Δt AR, and D AR Values on the AR Response
What is remarkable in the review of the AR protocols is that the single priming dose d AR is, in more than 90% of reports, lower than 0.05 Gy but higher than 0.001 Gy. It is well documented that the oxidative stress caused by 1 Gy X- or γ-rays simultaneously induces about 10 000 base damage (BD), 1000 DNA single-strand breaks (SSB), and 40 DNA double-strand breaks (DSB) per human diploid cell. These DNA damage induction rates are not dependent on the radiosensitivity status of the cells.52 Consequently, the priming doses d AR generally induce less than 500 BD, 50 SSB, and 2 DSB per cell. These numbers correspond to a significant oxidative stress much lower than that induced by the spontaneous DNA damage background usually observed in human radioresistant cells.53 Hence, such amounts of DNA damage cannot affect cell survival nor genomic instability significantly. By contrast, in radiosensitive cells, the oxidative stress due to spontaneous genomic instability causes a low but significant amount of DNA damage, larger than that observed in radioresistant cells.53 Hence, only in radiosensitive cells, the spontaneous oxidative stress added to that caused by the priming dose d AR may result in a significant amount of DNA damage: If a certain level of oxidative stress is required for the AR occurrence, the AR phenomenon should preferentially occur in radiosensitive cells.
The average repair half-times of BD, SSB, and DSB are about 5 to 10 minutes, 10 to 20 minutes, and 50 to 60 minutes, respectively.52 The review of the AR protocols reveals that Δt AR is strongly dependent on the d AR and D AR values and generally greater than 1 hour but less than 24 hours. In radioresistant cells, 24 hour postirradiation corresponds to a complete repair of all the DNA damage induced by d AR. By contrast, in radiosensitive cells, it is possible that some DSB may remain unrepaired or misrepaired after d AR + Δt AR. The DNA repair and signaling proteins may be therefore still activated during and after d AR + Δt AR. Hence, as hypothesized earlier and as the literature suggests AR should be much less frequent in radioresistant cells since d AR + Δt AR does not produce any significant biological effect before the exposure to D AR.
In the great majority of cases, D AR is higher than 0.5 Gy and frequently taken as 2 Gy, which corresponds to a high dose (a dose of 2 Gy per session is generally delivered in radiotherapy). A dose of 2 Gy generates a surviving fraction at 2 Gy (SF2) ranging between 1% to 10%, 10% to 60%, and 60% to 80% for the hyperradiosensitive, moderately radiosensitive, and radioresistant human cells, respectively (Table 1).53–55 When reviewing the literature, AR was not observed in cells with SF2 lower than 10% and larger than 60%, which supports again that AR preferentially occurs in cells showing moderate radiosensitivity. This conclusion is also in agreement with the fact that radioresistance is a “bounded notion”: Cells cannot repair more DNA damage when DNA repair is already complete and SF2 does not exceed 80% in human cells.53-55 Conversely, if cells are hyperradiosensitive, the amount of cell death or mutations is so high (the effect of d AR + Δt AR is already deleterious) that the biological consequences of an exposure to D AR is irreversible. Finally, it is noteworthy that a condition added to the d AR + Δt AR + D AR protocol to observe AR is that D AR should be higher than d AR. Indeed, there are very few examples, if any, of an occurrence of AR with D AR < d AR for 2 reasons, at least: (1) D AR alone produces hazardous or even lethal effect. An additional dose would simply produce additive effect on cells; (2) the notion of stimulus at the basis of AR to reduce the effect of a hazardous dose would become irrelevant if D AR < d AR.
Table 1.
Major Radiobiological Characteristics of the 3 Groups of Radiosensitivity as Defined in the study by Ryan et al.49
| Radiosensitivity Group | Clinical Features | Examples | Cellular Surviving Fraction at 2 Gy (%) | ATM Nucleo-Shuttling Features | AR Occurrence |
|---|---|---|---|---|---|
| Group I |
|
– | 60-80 | Fast | Yes but with high D AR |
| Group II |
|
BRCA1+/−
P53+/− radiotherapy responders |
10-60 | Delayed | Yes |
| Group III |
|
ATM−/−
LIG4−/− radiotherapy overresponders |
1-10 | Either absent (ATM−/−) or normal but with a gross DSB repair defect (eg, LIG4−/−) | No |
Abbreviations: AR, adaptive response; DSB, double-strand break.
What are the biological or clinical relevance of the d AR, Δt AR, and D AR values? In fact, the d AR + Δt AR + D AR scenario does not correspond to any current clinical, occupational, or environmental situation. One can however evoke a tumor positioning CT scan imaging (with a dose of the same order as d AR) followed by a radiotherapy session (with a dose of the same order as D AR). Nevertheless, even in this specific case, while AR may lead to decrease the radiosensitivity of healthy tissues, it would also decrease the radiosensitivity of the tumor, impacting therefore on the antitumor efficiency of the radiotherapy. Such scenario cannot be considered as a benefit for radiotherapy. It is noteworthy that most of reports about AR do not evoke the clinical relevance of the d AR + Δt AR + D AR scenario.
The Importance of the Cellular Model for the AR Occurrence and its Extent
From all the AR reports cited above, 2 remarks can be made:
In the cases of circulating lymphocytes, all the donors are not necessarily AR positive and the ratio of AR positive/negative donors varies with the reports. No genetic explanation is provided.
In all, the AR-positive human cells different from circulating lymphocytes listed from the 1980s, more than 50% are tumor cell lines, more than 30% are transformed or immortalized cells, and more than 75% are known to be radiosensitive.
These remarks consolidate the abovementioned conclusion that the occurrence of AR is favored when cells elicit abnormally high level of genomic instability and/or radiosensitivity. For example, the reduction in the mutations frequency caused by AR is better measurable in a cellular model in which deleterious mutations are already present (like in immortalized or tumor cell lines).51 In the case of human radioresistant primary fibroblasts showing AR, AR occurs when D AR is equal to or greater than 4 Gy. At such dose, the micronuclei yield is measurable even in radioresistant cells.48 However, if the level of genomic instability and/or radiosensitivity is too high, AR cannot alleviate irreversible damage. When cell survival was used as an endpoint, Raaphorst and Boyden showed that the hyperradioresistant Sk-Mel-3 do not elicit AR.16 Again, in the same report, the hyperradiosensitive HT144 tumor cell lines do not show AR since the effect of d AR + Δt AR is already deleterious. Altogether, these examples suggest that AR preferentially occurs in cells showing an intermediate radiation response. The AR can however occur with radioresistant cases but with nonbiologically relevant doses.48
Individual Radiosensitivity and Its Potential Link With AR
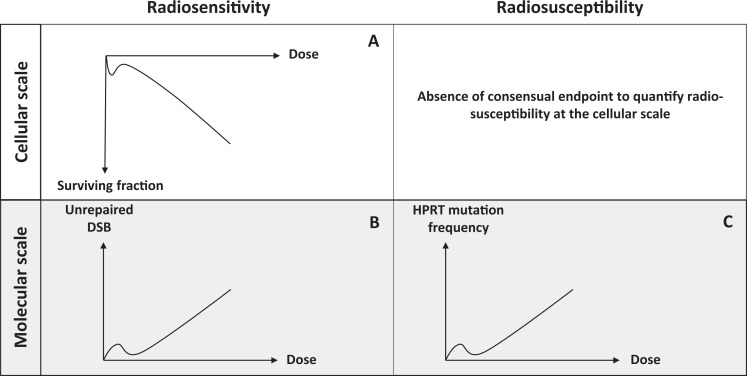
Can one predict the AR occurrence and the AR extent from the radiosensitivity status? The term “radiosensitivity” was historically defined as the proneness to radiation-induced adverse tissue events (notably after radiotherapy) and related to noncancer effects attributable to cell death. However, the term “radiosensitivity” is still used also for describing the radiation-induced cellular transformation and cancer52 (Figure 2). It must be stressed here that there is increasing evidence that the risk of radiation-induced tissue reactions and the risk of radiation-induced cancer are clearly different. For example, the Li-Fraumeni syndrome caused by heterozygous mutations of p53 is associated with high cancer risk but not with severe postradiotherapy adverse tissue events. Conversely, homozygous ATM (note 2) mutations are associated with both fatal adverse tissue events after radiotherapy and high cancer risk (lymphomas). Hence, to avoid any confusion, we recently proposed to describe radiation-induced transformation and cancer by the term “radiosusceptibility”52 (Figure 2).
Figure 2.
Schematic illustration of the hypersensitivity to low dose (hyperradiosensitivity, HRS) through the difference between radiosensitivity and radiosusceptibility. Cell survival (A) or unrepaired double-strand break (DSB; B) are the most relevant endpoints to quantify radiosensitivity (risk of tissue reactions attributable to cell death). Yield of hypoxanthine-guanine phosphoribosyl transferase (HPRT) mutations (C) is one of most relevant endpoints to quantify radiosusceptibility (risk of radiation-induced cancer). It is noteworthy that the endpoint that quantifies cell transformation is still not consensual.
What are the relationships between radiosensitivity and radiosusceptibility? Ataxia telangiectasia (AT) caused by homozygous mutations of the ATM gene is considered as the syndrome associated with the highest radiosensitivity observed in humans and characterized by fatal adverse tissue events after radiotherapy. The AT patients also had a very high risk of lymphomas. However, AT is a rare syndrome with an incidence of 1/100 000. There are other well-characterized syndromes associated with radiosensitivity. Some are characterized with high risk of cancer (eg, Nijmegen breakage syndrome) or with severe neurodegeneration and accelerated aging (eg, Hutchinson-Gilford progeroïd syndrome), suggesting that radiosensitivity is not necessarily linked systematically to cancer proneness and radiosusceptibility.52 Among the syndromes associated with both radiosensitivity and cancer proneness, the following rule is observed: The higher the incidence of syndromes, the less radiosensitive they are. For example, the BRCA1 and BRCA2 heterozygous mutations whose incidence is about 1/1000 lead to much less radiosensitivity than homozygous mutations of ATM whose incidence is 1/100 000). Conversely, these 3 cited mutations confer similar high risk of breast cancer, ovary cancer, and lymphoma, respectively.52 Hence, unlike BRCA1 and BRCA2 cases, AR may not occur in AT cells if radiosensitivity is chosen as an endpoint, while AR may be measurable in AT, BRCA1, and BRCA2 cells if mutations frequency (radiosusceptibility) is chosen as an endpoint. This last example illustrates the importance of the choice of both cellular models and endpoints in the study of AR.
Links Between Hyperradiosensitivity to Low Doses and AR
First described by Lambin et al56 and Marples and Joiner,57 the hyperradiosensitivity (HRS) to low-dose phenomenon results in a significant reduction in clonogenic cell survival, increase in chromosome breaks, micronuclei, and unrepaired DSB after a single low-dose d HRS taken between 1 and 800 mGy.58 The HRS phenomenon is not only observed with cell death and radiosensitivity-related endpoints but also concerns cellular transformation and genomic instability as well. Actually, HRS was observed when hypoxanthine-guanine phosphoribosyl transferase (HPRT) mutations were taken as an endpoint through an excess of mutations between 1 and 500 mGy.59 Hence, HRS cannot kill tumorigenic cells specifically, as it was hypothesized elsewhere,60 and this phenomenon can produce excess of cellular transformation (radiosusceptibility) and/or excess of cell death (radiosensitivity)59 (Figure 2). Besides, the maximal HRS response extent observed in human cells was reported to be limited to 25% cell survival: Even if HRS would kill specifically tumorigenic cells, its action would be limited to a minority of irradiated cells.61
The maximal HRS effect has been reported at a dose d HRSmax taken between 0.1 and 0.8 Gy (the most frequent d HRSmax is 0.2 Gy). The doses d HRS are supposed to produce a biological effect equivalent to a dose 5 to 10 times higher.58,61 More interestingly, similarly to AR, HRS has been generally observed in cells showing moderate radiosensitivity but neither in radioresistant nor in hyperradiosensitive cells.29,49,62 Furthermore, d HRS is of the same order as the d AR ranges described earlier although slightly higher (d AR ≤ d HRS). Ryan et al reported that, among 7 human tumor cell lines, 4 were found both AR- and HRS positive. Furthermore, in the same report, the 3 other cell lines were radioresistant and HRS negative but showed AR with high D AR values, suggesting that cells can be AR positive without being necessarily HRS positive.49 Altogether, the literature suggests that:
Cells that are very radioresistant do not show HRS and AR.
Cells that are hyperradiosensitive to high dose do not show HRS and AR.
All the HRS positive cells are AR positive. By contrast, all the AR positive cells are not necessarily HRS positive.
Both HRS and AR extents are maximal for the cells showing intermediate radiosensitivity.
How to explain these conclusions?
The Theory of ATM Nucleo-Shuttling: A Mechanism Explaining Both AR and HRS?
To date, there is evidence that unrepaired DSBs are linked to cellular lethality, and misrepaired DSBs are linked to genomic instability and cellular transformation.52 In 2016, we proposed a mechanistic model of HRS based on the theory of the ATM nucleo-shuttling.63 The ATM protein kinase is a major protein involved in the molecular and cellular response to IR and particularly in the DSB signaling and repair pathways. In the frame of this theory, cytoplasm can be considered as a reservoir of ATM dimers composed of 2 trans-autophosphorylated ATM monomers. The IR-induced oxidative stress induces monomerization of ATM dimers: The resulting ATM monomers diffuse in the nucleus where they contribute to the DSB recognition through the phosphorylation of the H2AX histone (γH2AX).63 The γH2AX phosphorylation triggers DSB repair via the nonhomologous end joining (NHEJ) pathway, the major DSB repair pathway in humans. The presence of ATM in the nucleus also inhibits the repair of DSB via the MRE11-dependent recombination-like error-prone pathway that leads to misrepaired DSB and cancer proneness.63 From a collection of 117 cell lines showing a large spectrum of postradiotherapy adverse tissue reactions, any delay in the ATM nucleo-shuttling causes radiosensitivity (via nonrecognition or nonrepair of DSB via NHEJ) and/or radiosusceptibility (via the MRE11-dependent recombination-like error-prone pathway). A quantitative correlation was found between the maximal number of pATM foci assessed by immunofluorescence in the first hour postirradiation (pATMmax) and the severity of postradiotherapy tissue reaction (evaluated by common terminology criteria for adverse events (CTCAE) scale).53 From the theory of the ATM nucleo-shuttling, a 3-group individual radiosensitivity classification was proposed:
Group I: radioresistance and low cancer risk; fast ATM nucleo-shuttling; complete DSB repair,
Group II: moderate radiosensitivity and high cancer risk; delayed ATM nucleo-shuttling; incomplete DSB repair,
Group III: HRS and high cancer risk; gross DSB repair defect.
The theory of the ATM nucleo-shuttling also permitted to solve the linear-quadratic model by considering 2 types of lethal unrepaired DSB63:
The α-type DSBs are recognized by NHEJ pathway but remain unrepaired. If the dose is not high enough to induce a sufficient number of radiation-induced ATM monomers, DSBs will not be recognized. This situation likely concerns low doses. At high doses, the recognition rate is constant and dose-independent. It was shown that the number of α-type DSB is simply proportional to D.63
The β-type DSBs are not recognized by NHEJ pathway and therefore never repaired. These DSBs are not recognized because the ATM redimerization and the reassociation with cytoplasmic proteins prevent the ATM monomers to diffuse in the nucleus. This situation likely concerns high doses. It was shown that this effect increases proportionally with dose at the constant rate. Consequently, the number of β-type DSB is proportional to D.2,63
Interestingly, the theory of the ATM nucleo-shuttling was also shown to provide a relevant biological interpretation for the modified linear-quadratic model that describes the HRS phenomenon. In fact, at low doses, as specified earlier, the number of ATM monomers produced by IR in cytoplasm is reduced, more particularly in the radiosensitive group II cells, characterized by a significant delay in the ATM nucleo-shuttling. In this situation, few ATM monomers are able to diffuse in the nucleus. Consequently, less DSBs are recognized by NHEJ and will remain unrepaired. It was shown that if the radiation-induced DSBs are not recognized by NHEJ during the first hour postirradiation, they can also be recognized by the MRE11-dependent, error-prone recombination-like pathway.63 Consequently, HRS can result in an excess of cell death due to unrepaired DSB or an excess of genomic instability due to misrepaired DSB, in agreement with the literature.63 Furthermore, HRS is preferentially observed in the radiosensitive group II cells that are characterized by a delay in the ATM nucleo-shuttling. Hence, even if the number of IR-induced DSB is low at low dose, the number of unrepaired or misrepaired DSB and their biological impact is measurable.63
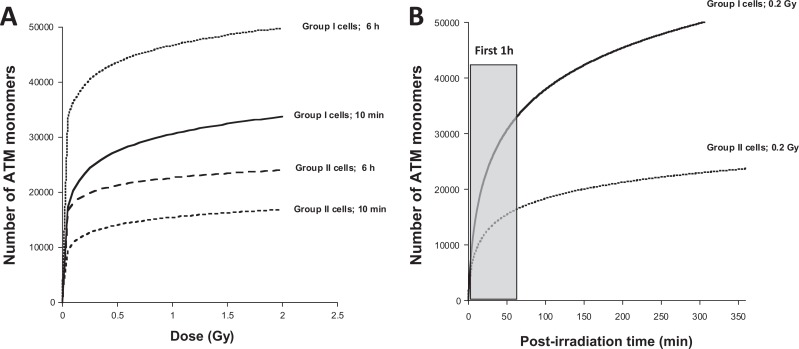
In the frame of the theory, N diff, the number of ATM monomers that diffuse in the nucleus as a function of dose D and time postirradiation t obeys the following formula (1)63:
| 1 |
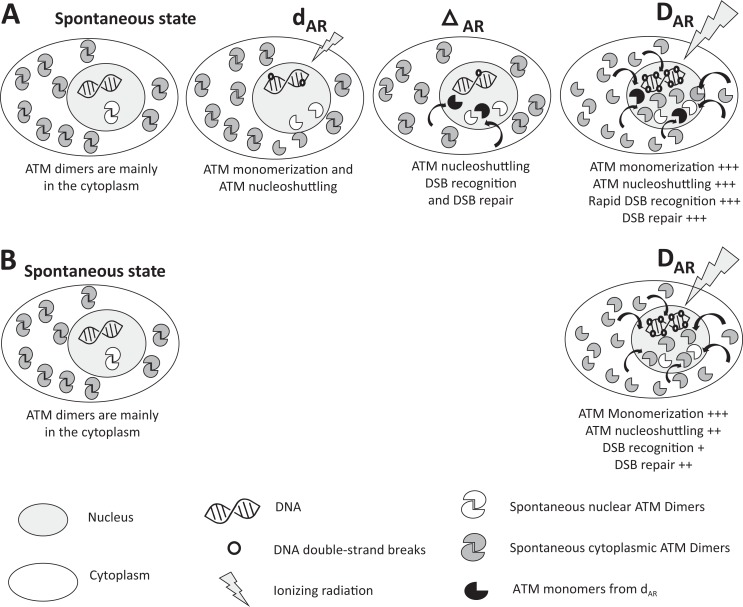
in which the nuclear membrane is characterized by a width L, a nucleus surface S, a permeability P and in which χmono is defined as the ATM monomers reassociation coefficient and Imono the number of IR-induced ATM monomers per Gy. By taking the numerical values validated in Bodgi and Foray from hundreds of human fibroblasts with different radiosensitivity, the diffusion of ATM monomers as a function of dose for 10 minutes and 6 hours was simulated for 2 representative group I and II cell lines (Figure 3A). Interestingly, the diffusion of ATM monomers increases very rapidly with dose. In group II cells, after 10 minutes postirradiation, a dose of 0.1 Gy leads to the diffusion of about 10 000 ATM monomers in the nucleus while a dose of 2 Gy leads to the diffusion of only 15 000 ATM monomers (Figure 3A). When the number of ATM monomers is observed as a function of time, more than 70% of ATM monomers are produced in the first hour postirradiation (Figure 3B). Because of the delay in ATM nucleo-shuttling, the radiosensitive group II cells show 2 times less ATM monomers in the nucleus than the radioresistant group I cells. For example, after 0.2 Gy (ie, average d AR) followed by 10 minutes, about 24 000 and 12 000 ATM monomers diffuse in the nucleus of group I and group II cells, respectively. The diffusion of ATM monomers continues with time and the numbers of ATM monomers in the nucleus become 40 000 and 20 000 at 6 hours postirradiation (ie, the average Δt AR) in the radioresistant group I and in the radiosensitive group II cells, respectively (Figure 3). Hence, after 0.2 Gy + 6 h, even if 80% of DSB are repaired (and therefore if 80% ATM monomers are inactivated by redimerization in the nucleus), the 20% remaining active monomers represent 8000 and 4000 ATM molecules in the nucleus of radioresistant group I and radiosensitive group II cells, respectively. A dose of 2 Gy (ie, average D RA) leads to the diffusion of about 30 000 and 16 000 additional ATM monomers in the nucleus after 10 minutes postirradiation, respectively (Figure 3). The 4000 ATM active monomers remaining in excess in the nucleus of the radiosensitive group II cells represent a supplement of about 25% (4000/16 000) ATM molecules that can significantly contribute to the DNA damage recognition with the ATM monomers induced directly by D RA (Figure 4). Such an excess of ATM monomers may permit the AR phenomenon to occur. In the frame of this model, if Δt AR is too short (less than 1 hour), the flux of ATM monomers may not be sufficient; if Δt AR is too long (more than 24 hours), the activity of monomers may become negligible. Hence, in the group II radiosensitive cells, d AR + Δt AR can produce a stimulus for DNA damage repair through an excess of ATM monomers in agreement with the literature. Finally, since the occurrence of HRS mainly depends on the number of ATM monomers in the first postirradiation hour, it is not surprising that HRS-positive cells are also AR positive. The theory of ATM nucleo-shuttling is therefore compatible with both HRS and AR phenomena (Figure 4).
Figure 3.
Number of ATM monomers that diffuse into the nucleus for radioresistant group I and radiosensitive group II cells. Data plots represent the numerical simulation derived from the formula (1) validated in the study by Bodgi and Foray.63 The following conditions were taken: S/L = 100·π·10−6 and χ mono Imono = 1.5 for group I cells and 3.8 for group II cells as proposed in the study by Bodgi and Foray.63 The Resulting formulas used here are: N I, diff (t, D) = 11 048 ln(1+1.5 Dt) and N II, diff (t, D) = 4221 ln(1+3.8 Dt) for group I and II cells, respectively. The A panel shows the simulated data as a function of the dose with the indicated repair times. The B panel shows the simulated data as a function of the repair time for a dose of 0.2 Gy. It is noteworthy that the first postirradiation hour is crucial for the hyperradiosensitivity (HRS) occurrence.
Figure 4.
General model for adaptive response (AR) based on the theory of the ATM nucleo-shuttling. The scenario d AR + Δt AR produces a significant excess of active ATM monomers in the nucleus before the exposure to D AR. As a result, more double-strand break (DSB) are recognized by non-homologous end joining (NHEJ) after d AR + Δt AR + D AR (panel A) than after D AR (panel B): radiosensitivity and radiosusceptibility decrease. However, it is noteworthy that AR is preferentially observed in radiosensitive (group II) cells. The AR is not observed in radioresistant (group I) cells since the repair of DSB induced by d AR + Δt AR is complete. The AR is not observed in hyperradiosensitive (group III) because the DNA damage caused by d AR + Δt AR are irreversible and their effect accumulates with that of D AR. It is noteworthy that hyperradiosensitivity (HRS) may occur after d AR according to the number of ATM monomers diffusing in the nucleus during the first postirradiation hour. The yield of spontaneous ATM monomers and dimers in the nucleus is likely to be much larger than their yield after exposure at d AR or D AR .
Conclusions
The AR phenomenon is an experimentally validated phenomenon observed in a variety of cellular models and with numerous experimental protocols that all obey the d AR + Δt AR + D AR irradiation scheme. It is not a technical artifact. It has been reported when radiosensitivity or radiosusceptibility are used as an endpoint. However, AR cannot be necessarily considered as beneficial since it may protect tumor or stimulate the transformation of healthy tissues. Furthermore, the AR occurrence strongly depends on the individual radiosensitivity/radiosusceptibility status: AR has been preferentially observed in cells from patients showing moderate radiosensitivity and high radiosusceptibility. Practically, to the notable exception of a tumor positioning CT scan that would precede a radiotherapy session after some hours, the d AR + Δt AR + D AR irradiation scheme is not encountered in clinic: AR phenomenon therefore remains a question relative to basic science. To date, the theory of the ATM nucleo-shuttling provides a relevant mechanistic explanation of the AR molecular and cellular events that reveals also the genetic status required for AR occurrence (Figure 4). The fact that the d AR + Δt AR + D AR irradiation scheme may produce sufficient amount of ATM monomers in the nucleus to contribute to respond to the dose D AR may evoke a kind of “vaccination”. However, the mechanistic model proposed here to explain the AR phenomenon is not related to the immune response to radiation. Further investigations of ATM nucleo-shuttling at low dose are needed to consolidate our model.
Supplemental Material
Supplemental_Data for Influence of Individual Radiosensitivity on the Adaptive Response Phenomenon: Toward a Mechanistic Explanation Based on the Nucleo-Shuttling of ATM Protein by Clément Devic, Mélanie L. Ferlazzo, and Nicolas Foray in Dose-Response
Acknowledgments
The authors thank Christiane Beaufrère for her technical help. The PhD thesis of C.D. is supported by Fibermetrix and Neolys Diagnostics companies. The postdoctoral fellowship of M.L.F is supported by the Centre National d’Etudes Spatiales (CNES). This work was supported by the Commissariat General à l’Investissement (Programmes Investissement d’avenir – INDIRA project).
Notes
Pasteur died only 3 months before the X-ray discovery in 1895.
The acronym ATM protein refers to Ataxia-telangiectasia mutated kinase as a key modulator in radiation-induced DNA damage repair.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Nicolas Foray  http://orcid.org/0000-0002-1282-1303
http://orcid.org/0000-0002-1282-1303
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Foray N. Victor despeignes, the forgotten pioneer of radiation oncology. Int J Radiat Oncol Biol Phys. 2016;96(4):717–721. [DOI] [PubMed] [Google Scholar]
- 2. Foray N. 1896-2016: la radiothérapie a 120 ans ! ou comment une discipline médicale a pu réunir Roentgen, Pasteur et les Frères Lumières [in French]. Cancer Radiother. 2016;20:820–823. [DOI] [PubMed] [Google Scholar]
- 3. Del Regato JA. Editorial: cell resistance incited by radiation. Am J Cancer. 1932;16(5):1246–1249. [Google Scholar]
- 4. Nogier T, Regaud C. Decroissance de la radiosensibilité des tumeurs malignes traitées par des doses successives et convenablement espacées des rayons X. Autoimmunisation contre les rayons [in French]. C R Acad Sci III. 1914;158(1):1711–1714. [Google Scholar]
- 5. Calabrese EJ, Baldwin LA. Radiation hormesis: its historical foundations as a biological hypothesis. Human Exp Toxicol. 2000;19(1):41–75. [DOI] [PubMed] [Google Scholar]
- 6. Calabrese EJ. Hormesis: a fundamental concept in biology. Microb Cell. 2014;1(5):145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calabrese EJ, Bachmann KA, Bailer AJ, et al. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222(1):122–128. [DOI] [PubMed] [Google Scholar]
- 8. Schwab M. Encyclopedia of Cancer. Heidelberg, Germany: Springer; 2011. [Google Scholar]
- 9. Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223(4636):594–597. [DOI] [PubMed] [Google Scholar]
- 10. Joiner MC, Lambin P, Marples B. Adaptive response and induced resistance. C R Acad Sci III. 1999;322(2-3):167–175. [DOI] [PubMed] [Google Scholar]
- 11. Southam CM., Ehrlich J. Effects of extract of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology. 1943;33:517–524. [Google Scholar]
- 12. Luckey TD. Hormesis With Ionizing radiation. New York, NY: CRC Press; 1980. [Google Scholar]
- 13. Calabrese EJ. Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem. 2008;27(7):1451–1474. [DOI] [PubMed] [Google Scholar]
- 14. Calabrese EJ. Model uncertainty via the integration of hormesis and LNT as the default in cancer risk assessment. Dose Response. 2015;13(4):1559325815621764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calabrese EJ, Shamoun DY, Hanekamp JC. The integration of LNT and hormesis for cancer risk assessment optimizes public health protection. Health Phys. 2016;110(3):256–259. [DOI] [PubMed] [Google Scholar]
- 16. Raaphorst GP, Boyden S. Adaptive response and its variation in human normal and tumour cells. Int J Radiat Biol. 1999;75(7):865–873. [DOI] [PubMed] [Google Scholar]
- 17. Bosi A, Olivieri G. Variability of the adaptive response to ionizing radiations in humans. Mutat Res. 1989;211(1):13–17. [DOI] [PubMed] [Google Scholar]
- 18. Wolff S, Afzal V, Wiencke JK, Olivieri G, Michaeli A. Human lymphocytes exposed to low doses of ionizing radiations become refractory to high doses of radiation as well as to chemical mutagens that induce double-strand breaks in DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1988;53(1):39–47. [DOI] [PubMed] [Google Scholar]
- 19. Wiencke JK, Afzal V, Olivieri G, Wolff S. Evidence that the [3H]thymidine-induced adaptive response of human lymphocytes to subsequent doses of X-rays involves the induction of a chromosomal repair mechanism. Mutagenesis. 1986;1(5):375–380. [DOI] [PubMed] [Google Scholar]
- 20. Shadley JD, Afzal V, Wolff S. Characterization of the adaptive response to ionizing radiation induced by low doses of X rays to human lymphocytes. Radiat Res. 1987;111(3):511–517. [PubMed] [Google Scholar]
- 21. Shadley JD, Wiencke JK. Induction of the adaptive response by X-rays is dependent on radiation intensity. Int J Radiat Biol. 1989;56(1):107–118. [DOI] [PubMed] [Google Scholar]
- 22. Shadley JD, Wolff S. Very low doses of X-rays can cause human lymphocytes to become less susceptible to ionizing radiation. Mutagenesis. 1987;2(2):95–96. [DOI] [PubMed] [Google Scholar]
- 23. Sanderson BJS, Morley AA. Exposure of human lymphocytes to ionizing radiation reduces mutagenesis by subsequent ionizing radiation. Mutat Res 1986;164(6):347–351. [DOI] [PubMed] [Google Scholar]
- 24. Sankaranarayanan K, von Duyn A, Loos MJ, Natarajan AT. Adaptive response of human lymphocytes to low-level radiation from radioisotopes or X-rays. Mutat Res. 1989;211(1):7–12. [DOI] [PubMed] [Google Scholar]
- 25. Kelsey KT, Memisoglu A, Frenkel D, Liber HL. Human lymphocytes exposed to low doses of X-rays are less susceptible to radiation-induced mutagenesis. Mutat Res. 1991;263(4):197–201. [DOI] [PubMed] [Google Scholar]
- 26. Shadley JD, Dai GQ. Cytogenetic and survival adaptive responses in G1 phase human lymphocytes. Mutat Res. 1992;265(2):273–281. [DOI] [PubMed] [Google Scholar]
- 27. Rigaud O, Moustacchi E. Radioadaptation to the mutagenic effect of ionizing radiation in human lymphoblasts: molecular analysis of HPRT mutants. Cancer Res. 1994;54(7 suppl):1924s–1928s. [PubMed] [Google Scholar]
- 28. Seong J, Suh CO, Kim GE. Adaptive response to ionizing radiation induced by low doses of gamma rays in human cell lines. Int J Radiat Oncol Biol Phys. 1995;33(4):869–874. [DOI] [PubMed] [Google Scholar]
- 29. Joiner MC, Lambin P, Malaise EP, et al. Hypersensitivity to very-low single radiation doses: its relationship to the adaptive response and induced radioresistance. Mutat Res. 1996;358(2):171–183. [DOI] [PubMed] [Google Scholar]
- 30. Ueno AM, Vannais DB, Gustafson DL, Wong JC, Waldren CA. A low, adaptive dose of gamma-rays reduced the number and altered the spectrum of S1- mutants in human-hamster hybrid AL cells. Mutat Res. 1996;358(2):161–169. [DOI] [PubMed] [Google Scholar]
- 31. Boothman DA, Meyers M, Odegaard E, Wang M. Altered G1 checkpoint control determines adaptive survival responses to ionizing radiation. Mutat Res. 1996;358(2):143–153. [DOI] [PubMed] [Google Scholar]
- 32. Salone B, Grillo R, Aillaud M, Bosi A, Olivieri G. Effects of low-dose (2 cGy) X-ray on cell-cycle kinetics and on induced mitotic delay in human lymphocyte. Mutat Res. 1996;351(2):193–197. [DOI] [PubMed] [Google Scholar]
- 33. Filippovich IV, Sorokina NI, Robillard N, Lisbona A, Chatal JF. Radiation-induced apoptosis in human tumor cell lines: adaptive response and split-dose effect. Int J Cancer. 1998;77(1):76–81. [DOI] [PubMed] [Google Scholar]
- 34. Redpath JL, Antoniono RJ. Induction of an adaptive response against spontaneous neoplastic transformation in vitro by low-dose gamma radiation. Radiat Res. 1998;149(5):517–520. [PubMed] [Google Scholar]
- 35. Cregan SP, Brown DL, Mitchel RE. Apoptosis and the adaptive response in human lymphocytes. Int J Radiat Biol. 1999;75(9):1087–1094. [DOI] [PubMed] [Google Scholar]
- 36. Chen Z, Sakai K. Enhancement of radiation-induced apoptosis by preirradiation with low-dose X-rays in human leukemia MOLT-4 cells. J Radiat Res. 2004;45(2):239–243. [DOI] [PubMed] [Google Scholar]
- 37. Hauptmann M, Haghdoost S, Gomolka M, et al. Differential response and priming dose effect on the proteome of human fibroblast and stem cells induced by exposure to low doses of ionizing radiation. Radiat Res. 2016;185(3):299–312. [DOI] [PubMed] [Google Scholar]
- 38. Zhao Y, Zhong R, Sun L, Jia J, Ma S, Liu X. Ionizing radiation-induced adaptive response in fibroblasts under both monolayer and 3-dimensional conditions. PLoS One. 2015;10(3):e0121289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ye S, Yuan D, Xie Y, Pan Y, Shao C. Role of DNA methylation in long-term low-dose gamma-rays induced adaptive response in human B lymphoblast cells. Int J Radiat Biol. 2013;89(11):898–906. [DOI] [PubMed] [Google Scholar]
- 40. Gajendiran N, Tanaka K, Kumaravel TS, Kamada N. Neutron-induced adaptive response studied in go human lymphocytes using the comet assay. J Radiat Res. 2001;42(1):91–101. [DOI] [PubMed] [Google Scholar]
- 41. Stoilov LM, Mullenders LH, Darroudi F, Natarajan AT. Adaptive response to DNA and chromosomal damage induced by X-rays in human blood lymphocytes. Mutagenesis. 2007;22(2):117–122. [DOI] [PubMed] [Google Scholar]
- 42. Shelke S, Das B. Dose response and adaptive response of non-homologous end joining repair genes and proteins in resting human peripheral blood mononuclear cells exposed to gamma radiation. Mutagenesis. 2015;30(3):365–379. [DOI] [PubMed] [Google Scholar]
- 43. Dieriks B, De Vos W, Baatout S, Van Oostveldt P. Repeated exposure of human fibroblasts to ionizing radiation reveals an adaptive response that is not mediated by interleukin-6 or TGF-beta. Mutat Res. 2011;715(1-2):19–24. [DOI] [PubMed] [Google Scholar]
- 44. Toprani SM, Das B. Radio-adaptive response of base excision repair genes and proteins in human peripheral blood mononuclear cells exposed to gamma radiation. Mutagenesis. 2015;30(5):663–676. [DOI] [PubMed] [Google Scholar]
- 45. Park HS, You GE, Yang KH, et al. Role of AKT and ERK pathways in controlling sensitivity to ionizing radiation and adaptive response induced by low-dose radiation in human immune cells. Eur J Cell Biol. 2015;94(12):653–660. [DOI] [PubMed] [Google Scholar]
- 46. Pretazzoli V, Salone B, Bosi A, Olivieri G. Variability of G(2) checkpoint sensitivity to low doses of X-rays (2 cGy): correlation with G(2) chromatid aberrations but not with an adaptive response. Mutagenesis. 2000;15(6):531–535. [DOI] [PubMed] [Google Scholar]
- 47. Mothersill C, Seymour CB, Joiner MC. Relationship between radiation-induced low-dose hypersensitivity and the bystander effect. Radiat Res 2002;157(5):526–532. [DOI] [PubMed] [Google Scholar]
- 48. Broome EJ, Brown DL, Mitchel RE. Dose responses for adaption to low doses of (60)Co gamma rays and (3)H beta particles in normal human fibroblasts. Radiat Res. 2002;158(2):181–186. [DOI] [PubMed] [Google Scholar]
- 49. Ryan LA, Seymour CB, Joiner MC, Mothersill CE. Radiation-induced adaptive response is not seen in cell lines showing a bystander effect but is seen in lines showing HRS/IRR response. Int J Radiat Biol. 2009;85(1):87–95. [DOI] [PubMed] [Google Scholar]
- 50. Toprani SM, Das B. Role of base excision repair genes and proteins in gamma-irradiated resting human peripheral blood mononuclear cells. Mutagenesis. 2015;30(2):247–261. [DOI] [PubMed] [Google Scholar]
- 51. Manesh SS, Sangsuwan T, Wojcik A, Haghdoost S. Studies of adaptive response and mutation induction in MCF-10A cells following exposure to chronic or acute ionizing radiation. Mutat Res. 2015;780:55–59. [DOI] [PubMed] [Google Scholar]
- 52. Foray N, Bourguignon M, Hamada N. Individual response to ionizing radiation. Mutat Res Rev. 2016;770(pt B):369–386. [DOI] [PubMed] [Google Scholar]
- 53. Granzotto A, Benadjaoud MA, Vogin G, et al. Influence of nucleoshuttling of the ATM protein in the healthy tissues response to radiation therapy: toward a molecular classification of human radiosensitivity. Int J Radiat Oncol Biol Phy. 2016;94(3):450–460. [DOI] [PubMed] [Google Scholar]
- 54. Joubert A, Zimmerman KM, Bencokova Z, et al. DNA double-strand break repair defects in syndromes associated with acute radiation response: at least two different assays to predict intrinsic radiosensitivity? Int J Radiat Bio. 2008;84(2):107–125. [DOI] [PubMed] [Google Scholar]
- 55. Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys. 1996;34(1):251–266. [DOI] [PubMed] [Google Scholar]
- 56. Lambin P, Marples B, Fertil B, Malaise EP, Joiner MC. Hypersensitivity of a human tumour cell line to very low radiation doses. Int J Radiat Biol. 1993;63(5):639–650. [DOI] [PubMed] [Google Scholar]
- 57. Marples B, Joiner MC. The response of Chinese hamster V79 cells to low radiation doses: evidence of enhanced sensitivity of the whole cell population. Radiat Res. 1993;133(1):41–51. [PubMed] [Google Scholar]
- 58. Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Biol Oncol Phys. 2001;49(2):379–389. [DOI] [PubMed] [Google Scholar]
- 59. Xue L, Yu D, Furusawa Y, Cao J, Okayasu R, Fan S. ATM-dependent hyper-radiosensitivity in mammalian cells irradiated by heavy ions. Int J Radiat Biol Oncol Phy. 2009;75(1):235–243. [DOI] [PubMed] [Google Scholar]
- 60. Tubiana M. Dose-effect relationship and estimation of the carcinogenic effects of low doses of ionizing radiation: the joint report of the Academie des Sciences (Paris) and of the Academie Nationale de Medecine. Int J Radiat Biol Oncol Phys. 2005;63(2):317–319. [DOI] [PubMed] [Google Scholar]
- 61. Thomas C, Martin J, Devic C, Diserbo M, Thariat J, Foray N. Impact of dose-rate on the low-dose hyper-radiosensitivity and induced radioresistance (HRS/IRR) response. Int J Radiat Biol. 2013;89(10):813–822. [DOI] [PubMed] [Google Scholar]
- 62. Marples B, Joiner MC. The elimination of low-dose hypersensitivity in Chinese hamster V79-379A cells by pretreatment with X rays or hydrogen peroxide. Radiat Res. 1995;141(2):160–169. [PubMed] [Google Scholar]
- 63. Bodgi L, Foray N. The nucleo-shuttling of the ATM protein as a basis for a novel theory of radiation response: resolution of the linear-quadratic model. Int J Radiat Biol. 2016;92(3):117–131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_Data for Influence of Individual Radiosensitivity on the Adaptive Response Phenomenon: Toward a Mechanistic Explanation Based on the Nucleo-Shuttling of ATM Protein by Clément Devic, Mélanie L. Ferlazzo, and Nicolas Foray in Dose-Response