Abstract
Background
Inflammatory arthritides including rheumatoid arthritis (RA), psoriatic arthritis (PsA) and ankylosing spondylitis (AS) are associated with increased risk of cardiovascular disease. This process may be driven by systemic inflammation, and the use of tumour necrosis factor (TNF) inhibitors could therefore potentially reduce cardiovascular risk by reducing this inflammatory burden. The aims of this study were to evaluate whether the risk of cardiovascular events (CVEs) in patients with inflammatory arthritis is associated with treatment with anti-TNF therapy, compared with other biologics or non-biologic therapy, and to compare the CVE risk between participants with RA, PsA and AS.
Methods
Data from consecutive participants in the Australian Rheumatology Association Database with RA, PsA and AS from September 2001 to January 2015 were included in the study. The Cox proportional hazards model using the counting process with time-varying covariates tested for risk of having CVEs, defined as angina, myocardial infarction, coronary artery bypass graft, percutaneous coronary intervention, other heart disease, stroke/transient ischaemic attack or death from cardiovascular causes. The model was adjusted for age, sex, diagnosis, methotrexate use, prednisone use, non-steroidal anti-inflammatory use, smoking, alcohol consumption, hypertension, hyperlipidaemia, diabetes and functional status (Health Assessment Questionnaire Disability Score).
Results
There were 4140 patients included in the analysis, totalling 19,627 patient-years. After multivariate adjustment, the CVE risk was reduced with anti-TNF use (HR 0.85, 95% CI 0.76–0.95) or other biologic therapies (HR 0.81, 95% CI 0.70–0.95), but not in those who had ceased biologic therapy (HR 0.96, 95% CI 0.83–1.11). After adjustment, no significant difference in CVE risk was observed between participants with RA and PsA (HR 0.92, 95% CI 0.77–1.10) or AS (HR 1.14, 95% CI 0.96–1.36).
Conclusions
Current biologic use was associated with a reduction in major CVEs. No reduction in CVE risk was seen in those who had ceased biologic therapy. After adjustment, the CVE risk was not significantly different between RA, AS or PsA.
Keywords: Biologicals, Cardiovascular disease, Rheumatoid arthritis, Psoriatic arthritis, Ankylosing spondylitis
Background
Inflammatory arthritides such as rheumatoid arthritis (RA), ankylosing spondylitis (AS) and psoriatic arthritis (PsA) impose a heavy burden of morbidity and mortality on populations worldwide. A significant component of this is the two-fold increased risk of cardiovascular events (CVEs) [1], with some evidence for increasing risk with longer disease duration [2–4]. It has been proposed that this is due to inflammatory processes driven by cytokines such as tumour necrosis factor (TNF), with a high inflammatory burden driving autoantibody production and apoptosis of endothelial cells to cause vascular damage [5] and a pro-thrombotic state [6].
The use of TNF inhibitors could therefore potentially reduce cardiovascular risk by controlling systemic inflammation. A recent study demonstrated that an RA cohort with disease onset after the year 2000 did not have an increased mortality risk compared to the general population, whereas those with disease onset prior to 2000 were at increased risk [7]. Several studies have demonstrated that treatment of inflammatory arthritis with TNF inhibitors is associated with an improvement in surrogate markers of cardiovascular health such as endothelial stiffness, biochemical lipid profile and carotid intima-media thickness [8–13].
There is conflicting evidence regarding clinical cardiovascular endpoints such as rate of myocardial infarction, stroke and cardiovascular-related death after treatment with biologics in patients with RA. Some studies report a lower risk of CVEs [14, 15], while others report no significant difference [16, 17]. Studies assessing cardiovascular risk in RA have been performed in locations including North America [14, 18, 19], Britain [20] and Sweden [21], but as yet no studies have been undertaken in the Australian context where there are stringent criteria for accessing biologic therapy. Furthermore, little research has been done to establish the effect of biologics on the CVE rate for inflammatory arthritis apart from RA. Thus, wider research is warranted in a range of arthritic conditions to examine whether biologic therapy is helpful beyond direct arthritic control in these patients.
The aim of this study was to determine whether the risk of CVEs in patients with RA, AS or PsA was associated with treatment with anti-TNF therapy, compared with other biologics or non-biologic therapy, and to compare the CVE risk between arthritis diagnoses.
Methods
The Australian Rheumatology Association Database (ARAD) is a national voluntary registry for patients with inflammatory arthritis (RA, AS, PsA and juvenile idiopathic arthritis). Details regarding the ARAD methodology have been described previously [22]. Briefly, participants with inflammatory arthritis complete self-reported questionnaires in paper or online format. Initially, these were completed biannually; however, from January 2014, the frequency of questionnaires was decreased to annually after the first 2 years of follow-up. The participant questionnaires include self-reported demographic details, current and past use of medications for arthritis, and current and past co-morbid medical conditions. Participants also complete patient-reported outcome measures including the Health Assessment Questionnaire Disability Score (HAQ)—a measure of functional status with scores ranging from 0 to 3 where higher scores indicate greater disability [23].
The majority of participants are referred by their treating rheumatologist (98.5%) and a small proportion is self-referred. Rheumatologists complete basic information at baseline including demographics and diagnosis. Cause of death is validated by data linkage to the Australian National Death Index, which provides verified International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) coding for cause of death [24]. Skilled data-entry personnel review inputs and correct errors, contacting participants for clarification as required.
Consecutive participants with RA, PsA or AS who had completed at least two separate ARAD questionnaires from database inception on 12 September 2001 to 28 January 2015 were included in the analysis. Demographic details, diagnosis, date of questionnaire, medications, medical history, HAQ score and, when applicable, cause of death were extracted from the ARAD on 28 January 2015. The primary outcome of interest was the composite rate of CVEs. CVEs were defined as any stable/unstable angina, myocardial infarction, coronary artery bypass graft, percutaneous coronary intervention, other heart disease (e.g. valvular), stroke/transient ischaemic attack or death from cardiovascular causes. This was in line with definitions commonly used in the literature [25–28]. Identification of CVEs, other than cardiovascular-related death, was based upon participants’ self-report. Based on ICD-10 codes obtained via data linkage with the Australian National Death Index, any cause of death in Chapter IX (Blocks I00–I99, “Diseases of the circulatory system”) was identified as a cardiovascular-related death and included in the composite measure of CVEs [24, 29].
Statistical methods
Survival analysis was conducted in SAS 9.4 using the Cox proportional hazards model and the counting process method to estimate hazard ratios (HRs) and corresponding 95% confidence intervals (95% CIs) for the rate of CVEs in patients who had anti-TNF biologic treatment, as compared to those with other biologic therapy or no biologic therapy. A repeated-events counting process model was utilised rather than a time-to-first-event model in order to account for the increased risk from multiple events during follow-up [30–32]. Participants who did not experience any CVE were right censored at the end of follow-up.
The main predictor of interest was biologic therapy use. The ARAD codes individual biologic therapies as current, previous, never or unknown, at each reported time point. For this analysis, biologic therapies were coded by conflation into anti-TNF (infliximab, etanercept, adalimumab, golimumab, certolizumab pegol) or other (anakinra, rituximab, abatacept, tocilizumab) to form the mutually exclusive groups of current anti-TNF use, current other biologic use, previous biologic use (any) or biologic-naïve. Data points where participants reported unknown biologic use were treated as missing and were excluded from the analysis. Medication use was coded as a time-varying variable to account for participants being put on different treatment across the longitudinal cohort study. Included participants were assumed to continue their reported biologic therapy for the interval between surveys.
Other participant characteristics included in the model as explanatory variables were age, sex, arthritis diagnosis, disease duration, alcohol usage and smoking status. Treatment status for non-steroidal anti-inflammatory drugs (NSAIDs), methotrexate and prednisone/prednisolone was coded as never, current, past or unknown. Co-morbid medical illnesses which are known cardiac risk factors (hyperlipidaemia, hypertension or diabetes) were also included as explanatory variables. These were self-reported in the ARAD as current, past, never or unknown. Those that were reported as current or past were coded as a positive history while those reported as never were coded as negative. Data points where participants reported an unknown history were treated as missing data and dropped from the analysis. The HAQ was included as a continuous variable. Univariate analyses were conducted and continuous variables were checked for linearity. Variables with a p value less than 0.25 in the univariate analysis were included in the multivariate model. Multi-collinearity in the multivariate model was evaluated using variance inflation factors (VIFs).
Multivariate analysis was performed using the backwards elimination method and the χ2 likelihood ratio test. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were reported using an α value of 0.05. The risk of CVEs was compared between RA, AS and PsA using the HR for each diagnosis from the final adjusted multivariate model. The results were reported in accordance with the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guidelines [33].
Results
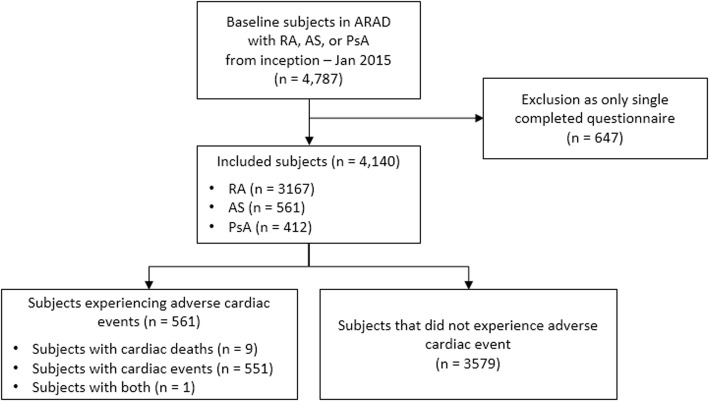
Between 2001 and 2015, there were 4787 participants enrolled in the ARAD with a diagnosis of RA, AS or PsA (Fig. 1). Participants with only a single completed questionnaire (n = 647) were excluded. Thus, 4140 participants were included in the analysis, totalling 32,844 completed questionnaires. Participant demographics at the time of enrolment in the ARAD are presented in Table 1. The median age was 56 years (interquartile range (IQR) 46–64 years), and 33.6% were male. The majority of participants had a diagnosis of RA (n = 3167, 76.5%), 561 (13.6%) had AS and 412 (10.0%) had PsA. The median time since diagnosis was 10 years (IQR 4–19 years) and the median (IQR) HAQ was 1.13 (0.50–1.75). Participants who had ever smoked regularly comprised 37.2% of the sample. In terms of alcohol use, 13.2% of participants were daily users, 54.4% occasional users and 32.4% non-users. Self-reported co-morbidities included hypertension (34.9%), hyperlipidaemia (19.1%) and diabetes (7.6%).
Fig. 1.
Flow diagram for participant inclusion from the ARAD. ARAD Australian Rheumatology Association Database, AS ankylosing spondylitis, PsA psoriatic arthritis, RA rheumatoid arthritis
Table 1.
Patient characteristics at ARAD enrolment (n = 4140)
| Median (interquartile range) | Number | Percentage | |
|---|---|---|---|
| Age (years) | 56 (46–64) | ||
| < 40 | 569 | 13.7 | |
| 40–49 | 779 | 18.8 | |
| 50–59 | 1182 | 28.6 | |
| 60–69 | 1067 | 25.8 | |
| ≥ 70 | 543 | 13.1 | |
| Sex | |||
| Male | 1393 | 33.6 | |
| Female | 2747 | 66.4 | |
| Disease duration (years) | 10 (4–19) | ||
| ≤ 5 | 1075 | 26.0 | |
| 6–10 | 833 | 20.1 | |
| 11–20 | 694 | 16.8 | |
| 21–30 | 536 | 12.9 | |
| > 30 | 986 | 23.8 | |
| Unknowna | 16 | 0.4 | |
| Diagnosis | |||
| RA | 3167 | 76.5 | |
| AS | 561 | 13.5 | |
| PsA | 412 | 10.0 | |
| Health Assessment Questionnaire Disability Scoreb | 1.13 (0.50–1.75) | ||
| Smoking regularly | |||
| Current or past | 1540 | 37.2 | |
| Never | 2129 | 51.4 | |
| Unknowna | 471 | 11.4 | |
| Alcohol consumption | |||
| Never | 1342 | 32.4 | |
| Sometimes | 2253 | 54.4 | |
| Every day | 545 | 13.2 | |
| History of co-morbid medical conditions | |||
| Diabetes | |||
| No | 3826 | 92.4 | |
| Yes | 313 | 7.6 | |
| Unknowna | 1 | 0.0 | |
| Hypertension | |||
| No | 2654 | 64.1 | |
| Yes | 1446 | 34.9 | |
| Unknowna | 40 | 1.0 | |
| Hyperlipidaemia | |||
| No | 3263 | 78.8 | |
| Yes | 791 | 19.1 | |
| Unknowna | 86 | 2.1 | |
| Angina | |||
| No | 3949 | 95.4 | |
| Yes | 171 | 4.1 | |
| Unknowna | 20 | 0.5 | |
| Myocardial infarction | |||
| No | 3984 | 96.2 | |
| Yes | 149 | 3.6 | |
| Unknowna | 7 | 0.2 | |
| Coronary artery bypass graft | |||
| No | 4084 | 98.6 | |
| Yes | 52 | 1.3 | |
| Unknowna | 4 | 0.1 | |
| Percutaneous coronary intervention | |||
| No | 4031 | 97.4 | |
| Yes | 105 | 2.5 | |
| Unknowna | 4 | 0.1 | |
| Other heart disease (e.g. valve disease) | |||
| No | 3914 | 94.5 | |
| Yes | 216 | 5.2 | |
| Unknowna | 10 | 0.2 | |
| Stroke/TIA | |||
| No | 4042 | 97.6 | |
| Yes | 92 | 2.2 | |
| Unknowna | 6 | 0.1 | |
ARAD Australian Rheumatology Association Database, AS ankylosing spondylitis, PsA psoriatic arthritis, RA rheumatoid arthritis, TIA transient ischaemic attack
aParticipant reports that they do not know, or are unsure
bRange 0–3 where a higher score indicates greater disability
Table 2 presents disease-modifying anti-rheumatic drug (DMARD) use at the time of enrolment in the ARAD: the majority of participants were recruited on current anti-TNF biologic therapy (56.8%), with some on alternative biologics (3.1%), and 36.8% of participants were biologic-naïve at ARAD enrolment. At baseline, 1776 (56.3%) participants with RA, 265 (64.5%) participants with PsA and 437 (78.0%) participants with AS were taking a biologic therapy. Current methotrexate use was reported by 55.6% of participants at enrolment, 39.0% were currently taking prednisone or prednisolone and 51.4% were currently taking NSAIDs.
Table 2.
DMARD usage at ARAD enrolment (n = 4140)
| Number | Percentage | |
|---|---|---|
| Biologic use | ||
| Never taken | 1525 | 36.8 |
| Currently taking anti-TNF biologics | 2350 | 56.8 |
| Currently taking other biologics | 128 | 3.1 |
| Abatacept | 32 | 0.8 |
| Anakinra | 7 | 0.2 |
| Rituximab | 66 | 1.6 |
| Tocilizumab | 23 | 0.6 |
| Previous use | 121 | 2.9 |
| Unknowna | 16 | 0.4 |
| Methotrexate status | ||
| Never taken | 977 | 23.6 |
| Currently taking | 2302 | 55.6 |
| Stopped taking | 856 | 20.7 |
| Unknowna | 5 | 0.1 |
| Prednisone/prednisolone status | ||
| Never taken | 1529 | 36.9 |
| Currently taking | 1613 | 39.0 |
| Stopped taking | 969 | 23.4 |
| Unknowna | 29 | 0.7 |
| NSAID status | ||
| Not currently taking | 2011 | 48.6 |
| Currently taking | 2129 | 51.4 |
ARAD Australian Rheumatology Association Database, DMARD disease-modifying anti-rheumatic drug, TNF tumour necrosis factor, NSAID non-steroidal anti-inflammatory drug
aParticipant reports that they do not know, or are unsure of the answer
The study period comprised a total of 19,627 patient-years. Therapy was primarily anti-TNF (12,555 patient-years, 64.0%) or other biologics (1963 patient-years, 10.1%), while 10.0% (1955 patient-years) had ceased biologic therapy and 15.9% (3116 patient-years) were biologic-naïve. Only 29 patient-years (0.1%) included unknown DMARD therapy. Across the study period, 552 participants (13.3%) experienced a composite cardiac event and 10 died secondary to cardiovascular causes, with only one of these 10 participants reporting a CVE during the study period before dying of a cardiovascular cause.
Univariate Cox proportional hazards regression analyses for the whole group showed that increased age, male gender, RA diagnosis, disease duration, greater disability (higher HAQ), ever smoking regularly, ever using methotrexate, current prednisone/prednisolone or NSAIDs, or a medical history of hypertension, hyperlipidaemia and diabetes were all significant predictors of CVEs at the 0.25 level of significance (Table 3). Use of biologic therapy, past but not current use of prednisone/prednisolone and any level of alcohol use were inversely associated with CVEs. Continuous variables of age and disease duration were evaluated for linearity, and there was no evidence of multi-collinearity.
Table 3.
Unadjusted univariate Cox proportional hazards regression for factors predicting cardiovascular events in patients with inflammatory arthritis (n = 4140)
| Factor | HR | 95% CI | p value |
|---|---|---|---|
| Increased age (years) | 1.05 | 1.06–1.07 | < 0.0001 |
| Greater disease duration (years) | 1.02 | 1.02–1.02 | < 0.0001 |
| Sex (males vs females) | 1.44 | 1.33–1.55 | < 0.0001 |
| Biologic use (referent: biologic naïve) | < 0.0001 | ||
| Current TNF biologics | 0.63 | 0.58–0.70 | < 0.0001 |
| Current other biologics | 0.69 | 0.60–0.80 | < 0.0001 |
| Stopped taking biologics | 0.97 | 0.85–1.10 | 0.59 |
| Diagnosis (referent: rheumatoid arthritis) | < 0.0001 | ||
| Ankylosing spondylitis | 0.61 | 0.53–0.70 | < 0.0001 |
| Psoriatic arthritis | 0.75 | 0.64–0.88 | 0.0004 |
| Methotrexate treatment (referent: never) | < 0.0001 | ||
| Currently taking methotrexate | 1.37 | 1.18–1.59 | < 0.0001 |
| Stopped taking methotrexate | 1.56 | 1.34–1.82 | < 0.0001 |
| Prednisone/prednisolone treatment (referent: never) | < 0.0001 | ||
| Currently taking prednisone | 1.35 | 1.22–1.49 | < 0.0001 |
| Stopped taking prednisone | 0.85 | 0.76–0.95 | 0.003 |
| NSAID treatment vs not currently taking | 1.19 | 1.10–1.28 | < 0.0001 |
| Smoking regularly ever | 1.50 | 1.39–1.62 | < 0.0001 |
| Alcohol use (referent: never) | < 0.0001 | ||
| Sometimes | 0.64 | 0.59–0.69 | < 0.0001 |
| Every day | 0.85 | 0.76–0.95 | 0.01 |
| Hypertension (referent: no) | < 0.0001 | ||
| Positive history for hypertension | 2.21 | 2.04–2.41 | < 0.0001 |
| Hyperlipidaemia (referent: no) | < 0.0001 | ||
| Positive history for hyperlipidaemia | 2.39 | 2.22–2.59 | < 0.0001 |
| Diabetes (referent: no) | < 0.0001 | ||
| Positive history for diabetes | 1.98 | 1.80–2.18 | < 0.0001 |
| Higher HAQa | 1.83 | 1.74–1.92 | < 0.0001 |
HR hazard ratio, CI confidence interval, HAQ Health Assessment Questionnaire Disability Score, NSAID non-steroidal anti-inflammatory drug, TNF tumour necrosis factor
aRange 0–3, where higher scores indicate greater functional impairment
Multivariate analysis for the whole group (Table 4) found that, following adjustment for potential confounders, compared to the biologic-naïve, the CVE risk was reduced with anti-TNF use (HR 0.85, 95% CI 0.76–0.95) as well as use of other biologic therapies (HR 0.81, 95% CI 0.70–0.95), but was not reduced when biologic use was ceased (HR 0.96, 95% CI 0.83–1.11). After adjustment, no significant difference in the CVE rate was observed between RA and PsA (HR 0.92, 95% CI 0.77–1.10) or AS (HR 1.14, 95% CI 0.96–1.36). Co-morbid hypertension, hyperlipidaemia and diabetes were all significant positive predictors of major adverse CVEs, as were increased age, male sex, ever smoking regularly, greater disability (higher HAQ) and current treatment with methotrexate or current use of NSAIDs. Alcohol use was associated with a decreased risk of CVEs. After adjusting for other variables, disease duration was not a significant predictor of major adverse CVEs.
Table 4.
Multivariate Cox proportional hazards regression for factors predicting cardiovascular events in patients with inflammatory arthritis (n = 4140)
| Factor | HR | 95% CI | p value |
|---|---|---|---|
| Increased age (years) | 1.05 | 1.05–1.06 | < 0.0001 |
| Sex (males vs females) | 1.72 | 1.57–1.88 | < 0.0001 |
| Biologic use (referent: biologic naïve) | 0.006 | ||
| Current TNF biologics | 0.85 | 0.76–0.95 | |
| Current other biologics | 0.81 | 0.70–0.95 | |
| Stopped taking biologics | 0.96 | 0.83–1.11 | |
| Diagnosis (referent: rheumatoid arthritis) | 0.18 | ||
| Ankylosing spondylitis | 1.14 | 0.96–1.36 | |
| Psoriatic arthritis | 0.92 | 0.77–1.10 | |
| Methotrexate treatment (referent: never) | 0.0001 | ||
| Currently taking methotrexate | 1.08 | 0.90–1.29 | |
| Stopped taking methotrexate | 1.28 | 1.07–1.53 | |
| Prednisone/prednisolone treatment (referent: never) | 0.02 | ||
| Currently taking prednisone/prednisolone | 0.96 | 0.85–1.08 | |
| Stopped taking prednisone/prednisolone | 0.86 | 0.76–0.97 | |
| NSAID treatment vs not currently taking | 1.22 | 1.13–1.32 | < 0.0001 |
| Smoking regularly ever | 1.17 | 1.07–1.27 | 0.0003 |
| Alcohol use (referent: never) | < 0.0001 | ||
| Sometimes | 0.77 | 0.70–0.84 | |
| Everyday | 0.77 | 0.68–0.87 | |
| Hypertension (referent: no) | < 0.0001 | ||
| Positive history for hypertension | 1.27 | 1.16–1.39 | |
| Hyperlipidaemia (referent: no) | < 0.0001 | ||
| Positive history for hyperlipidaemia | 1.65 | 1.52–1.80 | |
| Diabetes (referent: no) | < 0.0001 | ||
| Positive history for diabetes | 1.28 | 1.16–1.42 | |
| Higher HAQa | 1.48 | 1.40–1.57 | < 0.0001 |
HR hazard ratio, CI confidence interval, HAQ Health Assessment Questionnaire Disability Score, NSAID non-steroidal anti-inflammatory drug, TNF tumour necrosis factor
aRange 0–3, where higher scores indicate greater functional impairment
Discussion
This study has demonstrated a reduction in CVEs associated with biologic use for both anti-TNF and other biologic agents in ARAD participants with RA, PsA or AS, compared with ARAD participants who were biologic-naïve. However, this protective effect for the CVE rate was not observed in those who had ceased using biologic agents. Previous studies have shown that people with any inflammatory arthritis have increased rates of both cardiovascular morbidity and cardiovascular mortality compared to the general population [4, 34–36]. However, there are few primary studies directly comparing event rates between different forms of inflammatory arthritis.
Our study explored the relationship between anti-TNF use and CVEs in the Australian context and was also able to examine three different types of inflammatory arthritis in the same cohort. A reduced risk of myocardial infarction for RA patients treated with anti-TNF agents compared with conventional DMARDs was also reported in a recently updated analysis of the British Society for Rheumatology Biologic Register (BSRBR-RA) [37]. The baseline characteristics of patients entered in the ARAD are similar to the biologic-exposed population in the BSRBR-RA [20]. A similar reduction in acute coronary syndrome events for patients with RA using anti-TNF therapy was also found in a recent Swedish cohort study [38]. A recent systematic review reported a decreased risk of CVEs in patients with RA treated with TNF inhibitors or with methotrexate, and an increased risk in those using glucocorticoids or NSAIDs [39]. This review also reported that treatment with systemic therapy decreased the risk of CVEs in patients with PsA or psoriasis. However, there was insufficient data to compare the CVE risk between individual therapies.
In the multivariate model, while current methotrexate use was not associated with any difference in CVE risk, those who had ceased methotrexate had an increased risk of CVEs compared to those who had never taken the medication. Conversely, participants who had ceased taking prednisone or prednisolone were at lower risk of CVEs compared with those who had never taken prednisone. The reasons for these associations are unclear, but there may be confounding by indication for these medications. There may also be confounders which are not accounted for, including socioeconomic factors which may influence the prescription of different therapies, or some associations may have occurred by chance.
Strengths of this study include the large database of prospective longitudinal data, which was fully utilised with the counting process method of survival analysis which counts multiple events, and contrasts with the time-to-first-event analyses which has been used in previous studies [17, 35, 40]. Our study also had a moderate mean follow-up time of 5 years, and made a direct comparison between several forms of inflammatory arthritis. Additionally, the continual reporting of participant biologic use at each questionnaire significantly reduced the potential for misclassification bias.
This article also has some limitations due to the type of study and the structure of the database. This is an observational cohort study with the choice of therapy being made by the rheumatologists and patients, and as such is only able to show an association and not causation. Therefore, there are two possible explanations for the reduction in the CVE rate. There may be an intrinsic causative benefit of biologic therapy theoretically due to anti-inflammatory properties. Alternatively, it may be due to selection bias or bias by indication: rheumatologists choose to prescribe biologics for healthier patients, or to patients with higher levels of education or socioeconomic status who are consequently at lower risk of cardiovascular disease. While it is possible that patients with higher levels of co-morbidities may not have been offered biologics given higher thresholds for general health before treatment, patients that qualify for subsidy under the Australian Pharmaceutical Benefits Scheme must have more severe or resistant disease—overall, the net direction of any bias is therefore unclear [22]. Furthermore, disease activity measures such as active joint counts or inflammatory markers are not collected in the ARAD. It was therefore not possible to account for disease activity in this analysis and it is possible that it is tighter disease control achieved by biologic therapy which led to a reduction of CVEs, rather than an intrinsic effect of the biologics themselves acting on vascular inflammation.
There were only 10 deaths from cardiovascular causes observed in our study, which is lower than that which might be expected from the general Australian population. The rate of cardiovascular death in Australia in 2015 was 151 per 100,000 persons [41]. Therefore, approximately 30 deaths might have been expected in our study which included a total of 19,627 patient-years of follow-up. This low mortality rate may reflect a recruitment bias in the ARAD—most participants are Caucasian and speak English as their first language, and approximately one third have a tertiary-level education. Higher socioeconomic status and education levels are associated with reduced risk of cardiovascular death. The results may not be generalisable to the broader population with these conditions.
Apart from cardiovascular death, in this study CVEs were identified through participant self-report and it is possible that there was under-reporting of events. It is not possible to directly compare the incidence of CVEs in our study with data for the general population in Australia due to differences in the definitions of CVEs used in the Australian Institute of Health and Welfare (AIHW) analysis of the AIHW National Hospital Morbidity Database and AIHW National Mortality Database [41].
The majority of ARAD participants have RA. The ARAD was founded in 2001 for the purpose of monitoring the benefits and safety of new therapies, particularly biologics. At that time in Australia, biologics were only subsidised by the Pharmaceutical Benefits Scheme for RA, so these patients made up the bulk of initial recruitment until biologics were subsidised for AS in 2004 and PsA in 2006 [22]. However, this should not materially affect the analysis, as comparison is between biologic therapies and biologic-naïve patients. We did not find any difference in the CVE risk between RA, PsA and AS, after adjustment for other risk factors. However, as the number of participants with PsA and AS is small relative to the number of RA participants, a false negative finding is possible. The prevalence of biologics use in the ARAD population is higher than would be expected for the Australian population of patients with these rheumatic diseases. This likely reflects recruitment bias as patients commencing biologic therapy were targeted in the early recruitment process.
Furthermore, we used a composite measure for biologic use due to the small numbers of patients treated with each individual agent. It was therefore not possible to ascertain whether there was any difference in the CVE risk between individual biologic therapies. Although the ARAD collects information on reasons for biologic cessation, it was difficult to isolate a single cause to explain the finding that the CVE risk in the group who had ceased using biologic agents was not significantly different from the biologic-naïve group. This could be because those who had ceased biologic therapy were generally resistant to biologic therapy and thus did not derive any improvement in either disease status or the CVE rate, or it could be because any protective effect from biologic use is not sustained after biologic cessation and participants returned to their previous level of cardiovascular risk. Medication use was self-reported and dosages of glucocorticoid and DMARDs were not collected. Furthermore, some participants reported they were unsure if they had certain medical conditions, or had taken some medications. However, this made up only a small proportion of data points, and is unlikely to have affected the overall results.
Conclusions
Current use of biologics, whether anti-TNF or another mechanism of action, is associated with a reduction in the CVE rate compared to the rate among people with inflammatory arthritis who are biologic-naïve. This event reduction was no longer observed in those who had ceased biologic use. There was no difference in the CVE risk between RA, PsA and AS. These findings support the hypothesis that control of systemic inflammation in these conditions may reduce the cardiovascular risk.
Acknowledgements
The authors would like to thank Jillian Patterson, Kolling Institute of Medical Research Statistics Clinic, Royal North Shore Hospital for assistance with the recommended models of statistical analyses. They would like to acknowledge the contributions of Joan McPhee, Vibhasha Chand, Lyndall Henderson and the ARAD Steering Committee with special thanks to Graeme Carroll and Claire Barrett. The authors also thank Australian rheumatologists and patients for contributing data to the ARAD.
Funding
PS is supported by a Commonwealth Government of Australia National Health & Medical Research Council (NHMRC) postgraduate scholarship. RB is funded by an NHMRC Senior Principal Research Fellowship.
The ARAD is currently supported by unrestricted educational grants administered through the Australian Rheumatology Association from AbbVie Pty Ltd, Pfizer Australia, Sanofi Australia, Celgene Australian & NZ and Bristol-Myers Squibb Australia Pty Ltd. Previous sponsorship for the ARAD included an NHMRC Enabling Grant (384330), Amgen Australia Pty Ltd, Aventis, AstraZeneca, Roche, Monash University and Cabrini Health. Infrastructure support for the ARAD was received from Cabrini Health, Monash University, Royal North Shore Hospital and the Australian Rheumatology Association.
Availability of data and materials
The datasets analysed during the current study are not publicly available due to Human Research Ethics Committee requirements, but are available from the corresponding author on reasonable request.
Abbreviations
- ARAD
Australian Rheumatology Association Database
- AS
Ankylosing spondylitis
- BSRBR-RA
British Society for Rheumatology Biologic Register
- CI
Confidence interval
- CVE
Cardiovascular event
- DMARD
Disease-modifying anti-rheumatic drug
- HAQ
Health Assessment Questionnaire Disability Score
- HR
Hazard ratio
- ICD-10
International Statistical Classification of Diseases and Related Health Problems, 10th revision
- IQR
Interquartile range
- NSAID
Non-steroidal anti-inflammatory drug
- PsA
Psoriatic arthritis
- RA
Rheumatoid arthritis
- TNF
Tumour necrosis factor
Authors’ contributions
JLL, PS and LM analysed and interpreted the data. JLL and PS drafted the manuscript. All authors were substantially involved in study concept and design, critically reviewed the manuscript and approved the final version to be published.
Ethical approval and consent to participate
Twenty ethics committees and organisations have granted approval for the ARAD across each state in Australia (New South Wales: Northern Sydney Local Health District, Cancer Council NSW; Victoria: Cabrini Health, Monash University, Royal Children’s Hospital, St Vincent’s Hospital, Cancer Council Victoria; Queensland: Queensland Government; South Australia: The South Australian Department of Health and Ageing, Women’s and Children’s Hospital SA Health Network; Western Australia: Department of Health WA, Fiona Stanley Hospital, Rockingham General Hospital, Royal Perth Hospital, Southern Metropolitan Health Service, Government of WA; Tasmania: Tasmania Health, University of Tasmania; Australian Capital Territory: ACT Health and Community Care). Approval has also been granted by the Australian Institute of Health and Welfare, the Australian Government Department of Health and the Department of Defence and Veterans’ Affairs, the cancer registry in each state (New South Wales, Victoria, Queensland, South Australia, Western Australia, Tasmania, Australian Capital Territory, Northern Territory) and the National Cancer Statistics Clearing House. All participants provide informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joshua L. Lee, Email: joshualawrence.lee@health.nsw.gov.au
Premarani Sinnathurai, Phone: 61 2 9463 1887, Email: Premarani.Sinnathurai@health.nsw.gov.au.
Rachelle Buchbinder, Email: rachelle.buchbinder@monash.edu.
Catherine Hill, Email: Catherine.Hill@sa.gov.au.
Marissa Lassere, Email: m.lassere@unsw.edu.au.
Lyn March, Email: Lyn.march@sydney.edu.au.
References
- 1.Perk J, De Backer G, Gohlke H, Graham I, Reiner Ž, Verschuren M, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 2.Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis. 2006;65:1608–1612. doi: 10.1136/ard.2005.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 4.Han C, Robinson DW, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33:2167–2172. [PubMed] [Google Scholar]
- 5.Ferraccioli G, Gremese E. Thrombogenicity of TNF alpha in rheumatoid arthritis defined through biological probes: TNF alpha blockers. Autoimmun Rev. 2004;3:261–266. doi: 10.1016/j.autrev.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Beinsberger J, Heemskerk JW, Cosemans JM. Chronic arthritis and cardiovascular disease: altered blood parameters give rise to a prothrombotic propensity. Semin Arthritis Rheum. 2014;44:345–352. doi: 10.1016/j.semarthrit.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Lacaille D, Avina-Zubieta JA, Sayre EC, Abrahamowicz M. Improvement in 5-year mortality in incident rheumatoid arthritis compared with the general population—closing the mortality gap. Ann Rheum Dis. 2017;76:1057. doi: 10.1136/annrheumdis-2016-209562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen J, Shang Q, Tam LS. Targeting inflammation in the prevention of cardiovascular disease in patients with inflammatory arthritis. Transl Res. 2016;167:138–151. doi: 10.1016/j.trsl.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Di Minno MN, Iervolino S, Zincarelli C, Lupoli R, Ambrosino P, Pizzicato P, et al. Cardiovascular effects of Etanercept in patients with psoriatic arthritis: evidence from the cardiovascular risk in rheumatic diseases database. Expert Opin Drug Saf. 2015;14:1905–1913. doi: 10.1517/14740338.2015.1111870. [DOI] [PubMed] [Google Scholar]
- 10.Choy E, Ganeshalingam K, Semb AG, Szekanecz Z, Nurmohamed M. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology (Oxford) 2014;53:2143–2154. doi: 10.1093/rheumatology/keu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters MJ, van Sijl AM, Voskuyl AE, Sattar N, Smulders YM, Nurmohamed MT. The effects of tumor necrosis factor inhibitors on cardiovascular risk in rheumatoid arthritis. Curr Pharm Des. 2012;18:1502–1511. doi: 10.2174/138161212799504786. [DOI] [PubMed] [Google Scholar]
- 12.Brezinski EA, Follansbee MR, Armstrong EJ, Armstrong AW. Endothelial dysfunction and the effects of TNF inhibitors on the endothelium in psoriasis and psoriatic arthritis: a systematic review. Curr Pharm Des. 2014;20:513–528. doi: 10.2174/138161282004140213123852. [DOI] [PubMed] [Google Scholar]
- 13.Daien CI, Duny Y, Barnetche T, Daures JP, Combe B, Morel J. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis. 2012;71:862–868. doi: 10.1136/annrheumdis-2011-201148. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg JD, Kremer JM, Curtis JR, Hochberg MC, Reed G, Tsao P, et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576–582. doi: 10.1136/ard.2010.129916. [DOI] [PubMed] [Google Scholar]
- 15.Bili A, Tang X, Pranesh S, Bozaite R, Morris SJ, Antohe JL, et al. Tumor necrosis factor alpha inhibitor use and decreased risk for incident coronary events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66:355–363. doi: 10.1002/acr.22166. [DOI] [PubMed] [Google Scholar]
- 16.Desai RJ, Rao JK, Hansen RA, Fang G, Maciejewski M, Farley J. Tumor necrosis factor-alpha inhibitor treatment and the risk of incident cardiovascular events in patients with early rheumatoid arthritis: a nested case-control study. J Rheumatol. 2014;41:2129–2136. doi: 10.3899/jrheum.131464. [DOI] [PubMed] [Google Scholar]
- 17.Solomon DH, Avorn J, Katz JN, Weinblatt ME, Setoguchi S, Levin R, et al. Immunosuppressive medications and hospitalization for cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3790–3798. doi: 10.1002/art.22255. [DOI] [PubMed] [Google Scholar]
- 18.Al-Aly Z, Pan H, Zeringue A, Xian H, McDonald JR, El-Achkar TM, et al. Tumor necrosis factor-alpha blockade, cardiovascular outcomes, and survival in rheumatoid arthritis. Transl Res. 2011;157:10–18. doi: 10.1016/j.trsl.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Solomon DH, Curtis JR, Saag KG, Lii J, Chen L, Harrold LR, et al. Cardiovascular risk in rheumatoid arthritis: comparing TNF-alpha blockade with nonbiologic DMARDs. Am J Med. 2013;126:730. doi: 10.1016/j.amjmed.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon WG, Watson KD, Lunt M, Hyrich KL, British Society for Rheumatology Biologics Register Control Centre Consortium. Silman AJ, et al. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2007;56:2905–2912. doi: 10.1002/art.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ljung L, Askling J, Rantapaa-Dahlqvist S, Jacobsson L, Group AS The risk of acute coronary syndrome in rheumatoid arthritis in relation to tumour necrosis factor inhibitors and the risk in the general population: a national cohort study. Arthritis Res Ther. 2014;16:R127. doi: 10.1186/ar4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchbinder R, March L, Lassere M, Briggs A, Portek I, Reid C, et al. Effect of treatment with biological agents for arthritis in Australia: the Australian rheumatology association database. Intern Med J. 2007;37:591–600. doi: 10.1111/j.1445-5994.2007.01431.x. [DOI] [PubMed] [Google Scholar]
- 23.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. World Health Organization. 2016. http://apps.who.int/classifications/icd10/browse/2016/en. Accessed 4 Jan 2018.
- 25.Gulati AM, Semb AG, Rollefstad S, Romundstad PR, Kavanaugh A, Gulati S, et al. On the HUNT for cardiovascular risk factors and disease in patients with psoriatic arthritis: population-based data from the Nord-Trøndelag Health Study. Ann Rheum Dis. 2016;75:819–824. doi: 10.1136/annrheumdis-2014-206824. [DOI] [PubMed] [Google Scholar]
- 26.Husted JA, Thavaneswaran A, Chandran V, Eder L, Rosen CF, Cook RJ, et al. Cardiovascular and other comorbidities in patients with psoriatic arthritis: a comparison with patients with psoriasis. Arthritis Care Res (Hoboken) 2011;63:1729–1735. doi: 10.1002/acr.20627. [DOI] [PubMed] [Google Scholar]
- 27.Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 2010;49:295–307. doi: 10.1093/rheumatology/kep366. [DOI] [PubMed] [Google Scholar]
- 28.Fowkes FGR, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841–848. doi: 10.1001/jama.2010.221. [DOI] [PubMed] [Google Scholar]
- 29.Mozaffarian D, Benjamin E, Go A, Arnett D, Blaha M, Cushman M, et al. AHA statistical update heart disease and stroke statistics—2015 update. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 30.UCLA: Statistical Consulting Group. How can I model repeated events survival analysis in PROC PHREG? https://stats.idre.ucla.edu/sas/faq/how-can-i-model-repeated-events-survival-analysis-in-proc-phreg/. Accessed 12 Aug 2016.
- 31.Montemezzani S, Muller S, Sbardella C. Recurrent event survival analysis. Swiss Federal Institute of Technology. 2011. https://stat.ethz.ch/education/semesters/ss2011/seminar/contents/presentation_10.pdf. Accessed 12 Aug 2016.
- 32.Thomas L, Reyes EM. Tutorial: survival estimation for Cox regression models with time-varying coefficients using SAS and R. J Stat Softw. 2014;61:1–23. doi: 10.18637/jss.v061.c01. [DOI] [Google Scholar]
- 33.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Peters MJ, van der Horst-Bruinsma IE, Dijkmans BA, Nurmohamed MT. Cardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritis. Semin Arthritis Rheum. 2004;34:585–592. doi: 10.1016/j.semarthrit.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Hagberg KW, Peng M, Shah K, Paris M, Jick S. Rates of cardiovascular disease and major adverse cardiovascular events in patients with psoriatic arthritis compared to patients without psoriatic arthritis. J Clin Rheumatol. 2015;21:405–410. doi: 10.1097/RHU.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathieu S, Motreff P, Soubrier M. Spondyloarthropathies: an independent cardiovascular risk factor? Joint Bone Spine. 2010;77:542–545. doi: 10.1016/j.jbspin.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Low ASL, Symmons DPM, Lunt M, Mercer LK, Gale CP, Watson KD, et al. Relationship between exposure to tumour necrosis factor inhibitor therapy and incidence and severity of myocardial infarction in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76:654–660. doi: 10.1136/annrheumdis-2016-209784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ljung L, Rantapää-Dahlqvist S, Jacobsson LTH, Askling J. Response to biological treatment and subsequent risk of coronary events in rheumatoid arthritis. Ann Rheum Dis. 2016;75:2087–2094. doi: 10.1136/annrheumdis-2015-208995. [DOI] [PubMed] [Google Scholar]
- 39.Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:480–489. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ljung L, Simard JF, Jacobsson L, Rantapaa-Dahlqvist S, Askling J, Anti-Rheumatic Therapy in Sweden Study Group Treatment with tumor necrosis factor inhibitors and the risk of acute coronary syndromes in early rheumatoid arthritis. Arthritis Rheum. 2012;64:42–52. doi: 10.1002/art.30654. [DOI] [PubMed] [Google Scholar]
- 41.Australian Institute of Health and Welfare. Cardiovascular Health Compendium. 2017. https://www.aihw.gov.au/reports/heart-stroke-vascular-disease/cardiovascular-health-compendium/data. Accessed 29 Apr 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are not publicly available due to Human Research Ethics Committee requirements, but are available from the corresponding author on reasonable request.