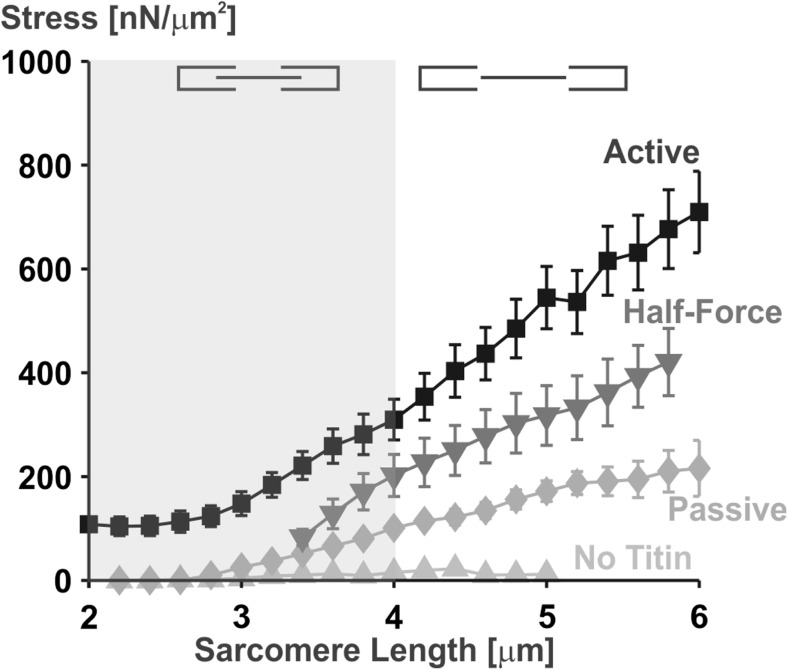
Fig. 8.
Stress (force/cross-sectional area) versus sarcomere length relationship for single rabbit psoas myofibrils stretched from an average sarcomere length of 2.0 μm to 6.0 μm. Myofibrils were stretched passively (Passive), actively (Active), actively from an average sarcomere length of 3.4 μm (Half-Force), and after deletion of titin (No Titin). Actin myosin filament overlap is lost at an average sarcomere length of about 4.0 μm (indicated by the shaded area). According to the cross-bridge theory, one would expect the forces beyond actin myosin filament overlap (non-shaded area) to be purely passive and the same for all conditions with intact titin filaments. However, the forces in the actively stretched myofibrils were substantially greater than those for the passively stretched myofibrils in the area where myofilament overlap was lost. Deactivation of selected myofibrils at an average sarcomere length of 5.0 μm did not result in a loss of force (results not shown), indicating that there was no remnant cross-bridge-based force at these lengths. Elimination of titin from the myofibrils abolished all passive and all active force in myofibrils, indicating that titin is not only an essential protein for passive force production but is absolutely essential for active force transmission from the cross-bridges to the Z-bands and for centering the myosin filaments in the sarcomere. Adapted from Leonard and Herzog (2010) with permission