Fig. 1.
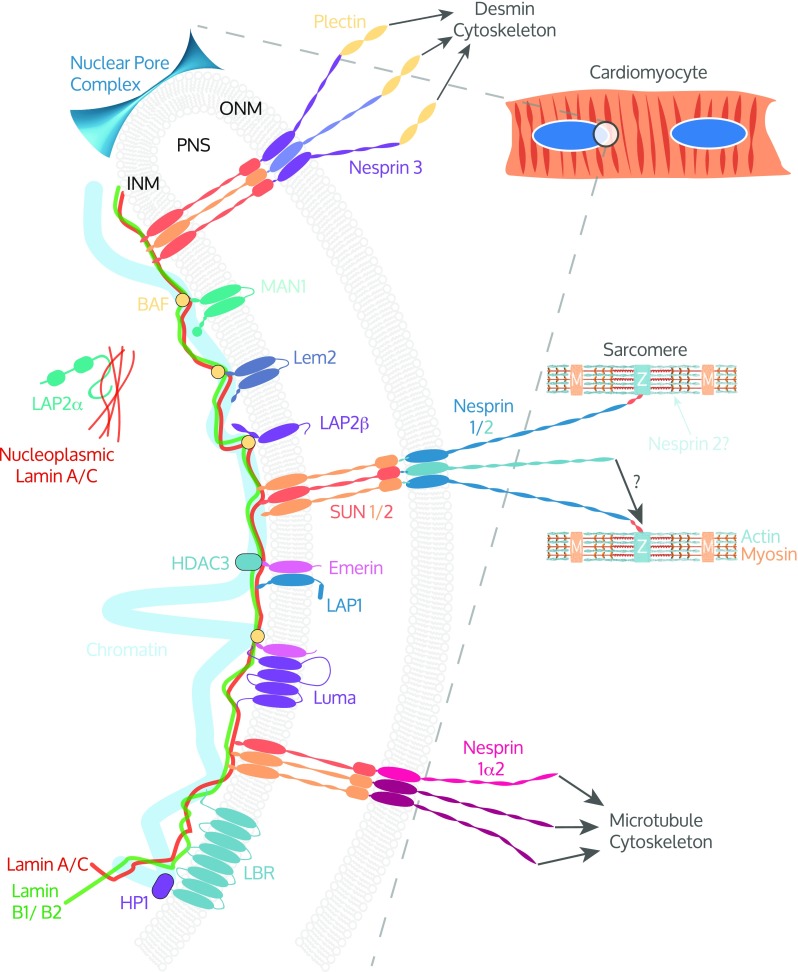
The cardiomyocyte LINC complex: Magnified view of cardiac myocyte nuclei (blue) in the circled region highlighting the nuclear envelope (NE) and linker between nucleoskeleton and cytoskeleton (LINC) complex. The LINC complex forms a continuous network of protein-protein interactions between the nuclear lamins; lamins A/C, B1, B2; and the various cytoskeletons. Inner nuclear membrane (INM) proteins SUN1 and SUN2 form heterotrimeric complexes that interact via their SUN domain with the KASH domain of the Nesprins that reside in the outer nuclear membrane (ONM). The giant isoforms of Nesprins 1 and 2 may directly link the NE to the sarcomere by interacting with the Z-disk (Z) or indirectly through intermediate binding partners(s). Nesprin 2 is reported at A/I junctions in the sarcomere. Nesprin 1α2 interacts with kinesin 1, thereby linking the NE to the microtubule cytoskeleton and Nesprin 3 indirectly links to the desmin intermediate filaments through plectin. Many other proteins are associated with the LINC complex that interact with lamins or SUN proteins including LAP2α, Emerin, MAN1, LEM2, Luma, and LAP1 and have been shown to play important roles in cardiomyocytes. Heterochromatin directly interacts with lamin A/C and indirectly with LEM domain proteins via barrier to autointegration factor (BAF). Emerin binds the histone modification enzyme histone deacetylase 3 (HDAC3). Lamin B receptor (LBR) interacts with lamin B and heterochromatin via heterochromatin protein 1 (HP1). LAP2α interacts with nucleoplasmic lamin A/C. PNS indicates perinuclear space and M, M-band
