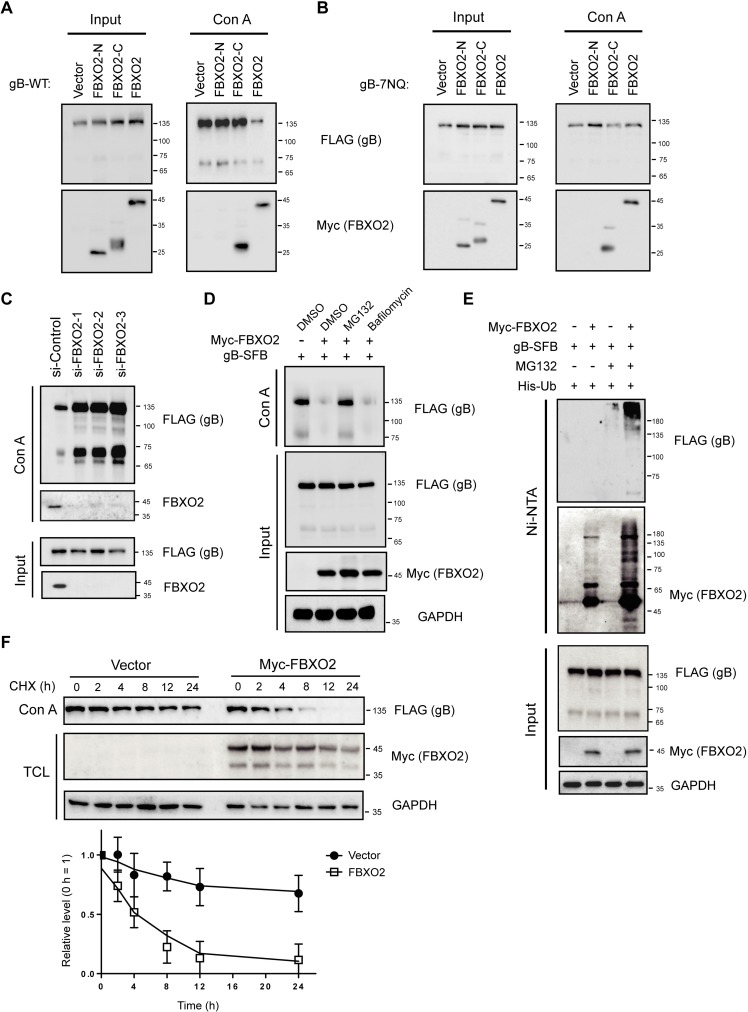
Fig 4. FBXO2 ubiquitinates and degrades N-linked glycosylated gB.
(A) FBXO2 reduces the level of glycosylated gB. HEK293T cells were co-transfected with gB, along with the FL, N-terminal or C-terminal FBXO2 constructs. 48 hrs later, the glycosylated proteins were enriched by Con A agarose pull-down, and the bound proteins and total cell lysates were immunoblotted with anti-FLAG and anti-Myc antibodies. (B) FBXO2 did not affect the total or glycosylated levels of the glycosylation-defective 7NQ gB mutant. The experiments were conducted as in (A). (C) CNE2 cells stably expressing gB were transfected with three siRNAs targeting FBXO2 or a scramble control siRNA, and 72 h later, the cells were harvested and subjected to Con A pull-down and WB. (D) Effects of MG132 and bafilomycin A1 on the levels of total and glycosylated gB. (E) FBXO2 ubiquitinates gB in vivo. HEK293T cells were transfected with His-ubiquitin (Ub), gB-SFB and Myc-FBXO2 or empty vector. The cells were treated with or without 20 μM MG132 for 6 h before harvest. 48 hrs post-transfection, the cells were lysed in 6 M guanidine-HCl buffer, the His-tagged ubiquitinated proteins were enriched by Ni-NTA (nickel-nitrilotriacetic acid) pull-down, and the bound proteins were eluted by SDS loading buffer and immunoblotted with antibodies as indicated. (F) HEK293T cells stably expressing gB were transfected with empty vector or Myc-FBXO2, and 36 h later the cells were treated with 50 μg/mL cycloheximide (CHX) to block protein synthesis and collected at the indicated time points. (top) Total cell lysates were analyzed by WB; (bottom) the degradation of gB was calculated by determining the relative quantification of gB from WB results. The mean value for vector-transfected cells at 0 h was normalized to a relative protein level of 100% (n = 3).