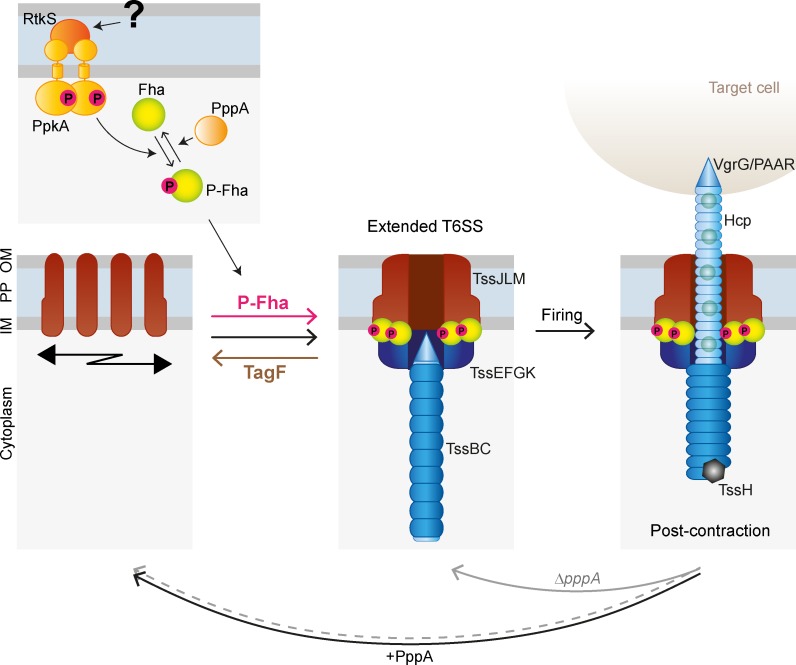
Fig 8. Schematic model for post-translational regulation of T6SS firing in S. marcescens.
Prior to assembly of the T6SS basal complex (membrane complex, TssJLM, plus baseplate complex, TssEGFK-VgrG), the membrane complex components are free to move around the cell. TagF inhibits assembly of the mature membrane complex which represents the site of baseplate docking. In response to an unknown signal, RtkS interacts with the periplasmic domain of PpkA, leading to PpkA autophosphorylation and phosphorylation of Fha, which is antagonised by PppA. Phosphorylated Fha multimerises and associates with the T6SS, overcoming TagF-mediated repression of basal complex formation. Next, the inner Hcp tube and surrounding TssBC sheath assemble on VgrG and TssE, respectively, and extend into the cytoplasm. This ‘extended’ T6SS is primed and ready to fire. During the firing event, the TssBC sheath contracts, propelling the Hcp-VgrG-PAAR puncturing structure out of the cell through the membrane complex and into a target cell. Effectors decorating the Hcp-VgrG-PAAR structure are released into a target cell, or else to the medium if no target is present. TssH then binds the contracted TssBC sheath and initiates depolymerisation, the basal complex disassembles and PppA dephosphorylates Fha. For a new round of firing to occur, in a wild type cell, TagF-mediated repression must again be overcome by phosphorylation of Fha, allowing time for the T6SS components to move and reassemble in a new spatial location. In the absence of PppA, Fha remains fully phosphorylated, leading to a tendency for the T6SS to immediately reassemble in the same place. When PpkA and TagF are both absent, the intrinsic ability of the system to assemble is observed but anti-bacterial activity is less efficient than with proper post-translational activation in place. IM, inner membrane; PP, periplasm; OM, outer membrane.