Figure 2.
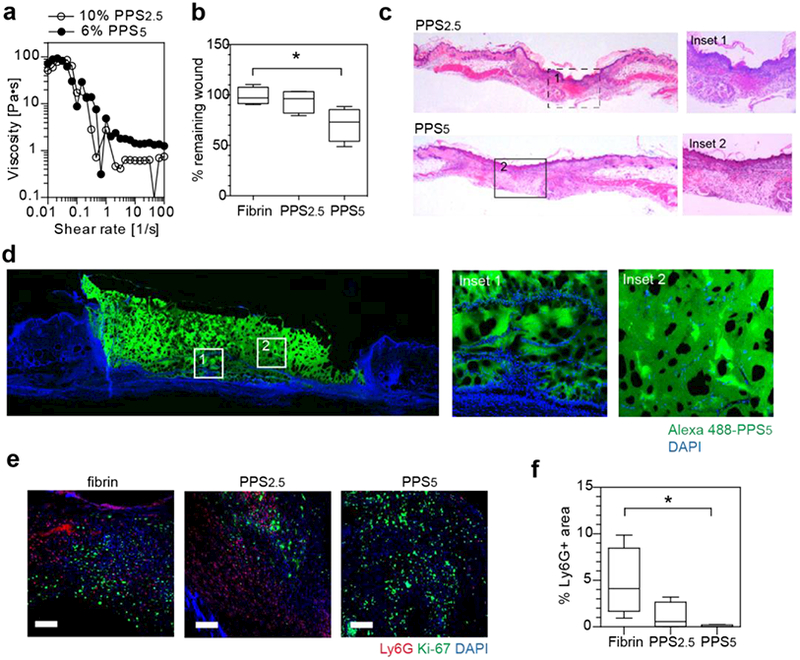
Injectable star-PEG-PPS hydrogels as biocompatible cutaneous wound scaffolds. a. star-PEG-PPS5 (6 w/v% in PBS) and star-PEG-PPS2.5 (10 w/v% in PBS) showed a similar viscosity and injectability. b. Wound closure comparision at day 3 in mouse splinted dermal excisional wound healing model among fibrin matrix (10 mg/mL fibrinogen), 10 w/v% star-PEG-PPS2.5, and 6 w/v% star-PEG-PPS5. c. H&E staining of day 7 mouse splinted dermal wounds, full length and inset showing wound bed center. d. Fluorescent imaging of day 7 mouse splinted dermal wound filled with Alexa 488-labeled star-PEG-PPS5. e. Immunohistochemical staining of day 7 neutrophil marker (Ly6G, red), proliferating cells (Ki-67, green), and nuclei (DAPI, blue) in wound beds dressed with fibrin matrix (10 mg/mL fibrinogen), 10 w/v% star-PEG-PPS2.5, or 6 w/v% star-PEG-PPS5. f. Quantification of images in e for positive Ly6G area. (n=4; one-way ANOVA, non-parametric, Tukey’s post test; Scale bar = 20 μm).
