Summary
Predictive tests, to refine the estrogen receptor assay, for the adjuvant treatment of breast cancer with tamoxifen and oral Selective Estrogen Receptor Degraders (SERDs) are required. A splice variant of the corepressor NCOR2, BQ2313636.1 predicts tamoxifen resistence to adjuvant tamoxifen and AZ9496, the first oral SERD, completes phase one studies.
In this issue of Clinical Cancer Research, Gong and colleagues(1) and Hamilton and colleagues (2) addressed the question: how can we improve upon the ER as a predictive test for adjuvant tamoxifen therapy and how can we advance therapeutics for breast cancer beyond tamoxifen, AIs, and the injectable SERD fulvestrant? Initial answers to these questions hold the potential to unravel the Gordian Knot of cancer complexity.
The estrogen receptor (ER)(3) is the key to the success or failure of adjuvant tamoxifen therapy. The translational research strategy(4,5) of long term adjuvant tamoxifen treatment increases survival for patients with ER positive breast cancer. However, acquired resistance to tamoxifen develops during continuous therapy and this resistance is unique. Laboratory studies demonstrate that ER positive breast tumors eventually grow in tamoxifen treated immune deficient mice. This is a demonstration of resistance to treatment. Nevertheless, retransplantation of tumors to new generations of immune deficient mice, demonstates that the breast cancer cells are actually dependant on tamoxifen for growth(6). Surprisingly, these same tumors will also grow with estrogen(6) treatment. This observation provided a scientific explanation for the clinical phenomenon of a withdrawal response following tamoxifen failure(7). Mechanisms for this dualist action of estrogen and tamoxifen on breast cancer growth have subsequently been deciphered(8). However, as with all cancers, the mechanisms of resistance are multifaceted.
The steroidal ER degrader (SERD) fulvestrant, binds to ER and the disrupted complex is targeted for ubiquitinylation and proteosomal distruction. The steroidal SERDs were first tested in the tamoxifen-stimulated immune deficient mouse breast cancer model(9). The overall laboratory conclusion for second line therapies following acquired tamoxifen resistence, was to use a SERD (fulvestrant) or an aromatase inhibitor to provide no estrogen signaling for the tumor to grow. Clinical trials subsequently demonstrated the veracity of the translational science(10). These treatment strategies became the standard of care.
Coregulatory molecules bind to the liganded ER complex either to enhance cell replication (coactivators) or prevent cell replication (corepressors)(11). The tamoxifen ER complex recruits dimerized NCOR2 to block growth. The finding(1) that a novel splice variant of NCOR2, BQ323637.1 (BQ), is present in some breast cancers is an interesting observation. The variant BQ dimerizes with NCOR2 thereby creating a flawed platform to recruit the necessary additional coregulatory proteins. This resistance mechanism is novel and has potential for clinical applications. Presumably, if the tamoxifen ER complex is not emasculated by recruitment of dimerized NCOR2, then the tamoxifen ER complex becomes stimulatory(Figure 1).
Figure 1.
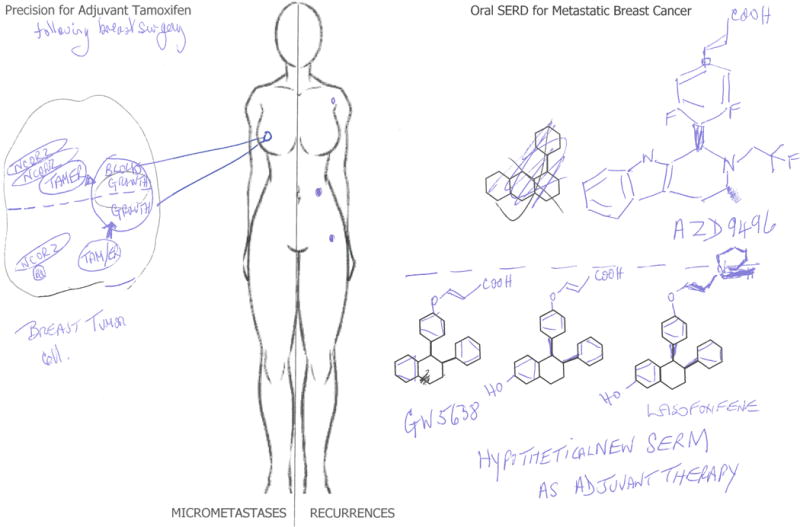
Tamoxifen is used for the long-term adjuvant treatment of ER positive breast cancer. The tamoxifen (or metabolite) ER complex requires dimerized NCOR2 to bind to ER and recruit other inhibitors of cells signaling to prevent breast cancer growth. A splice variant of NCOR2, BQ, binds to NCOR2 and prevents dimerization. This imperfect corepressor cannot bind to the tamoxifen ER complex. As a result, the BQ variant prevents the emasculation of the tamoxifen ER complex. Recurrence results. An alternative endocrine therapy to the injectable SERD fulvestrant, is an orally active SERD, to advance from the treatment of metastatic breast cancer (MBC) to long-term adjuvant therapy. The compound ASD9496 has completed a phase I study. However, side effects may retard studies as an adjuvant therapy. The structure of AZD9496 contains an acrylic acid antiestrogen sidechain that destroys the ER. It is interesting to reflect that this same sidechain on the known SERM GW5638 could be transferred to a SERM such as lasofoxifene with a proven record of positive SERM properties. Not only is there potential in the future for a new oral SERD but also an oral super SERD, that has enhanced healthcare benefits for our aging population when ER positive breast cancer develops.
Gong and coworkers(1), assemble 358 breast cancer cases that could be scored for BQ and were also ER positive. Despite this limitation, low and high nuclear BQ scores were used to predict overall survival or disease specific survival. High nuclear BQ undermine the antitumor actions of adjuvant tamoxifen and these data are highly significant over 20 years.
The new orally active SERD AZD9496(12) (Figure 1) is the first to complete a phase 1 clinical trial(2). The novel acrylic acid sidechain in AZD9496 is a common feature of a number of the new orally active SERDs(13). However, the medicinal chemistry has its origins in the Selective Estrogen Receptor Modulator (SERM) GW5638(14) (Figure 1). Dose escalation of AZD9496(2) up to 600mg twice daily, administered to 45 heavily pretreated patients (none of whom took tamoxifen) with ER positive/HER2 negative disease, showed one with stable disease at one year and one partial response. Adverse events (AEs) were: 7 patients with ≥ grade 3 AEs, 3 patients with dose limiting toxicity, and 1/3 of patients experiencing diarrhea and fatigue.
One advantage of AZD9496 is the finding that the oral SERD is effective against wildtype ER and mutant ER (D538G,Y537S) containing tumors(12). The mutant ERs (D538G, Y537S) are responble for closing helix 12 of the unliganded ER during AI adjuvant therapy, to D351 which produce an auto stimulatory unliganded ER complex that drives recurrence(15). This combined quality of oral activity and SERD action(12) against a known mechanisim AI resistance(15) are the necessary hallmarks for the success of a new oral SERD in the treatment of recurrent breast cancer during AI adjuvant therapy.
Nevertheless, the one thing we learned about the strategic use of tamoxifen was that when treatment was given to patients with metastatic breast cancer, everyone died within two to three years. By contrast, administration of tamoxifen, as a longterm (10 year), adjuvant therapy after surgery(4), significantly increases survival. Unfortunately, the limiting factor with longterm adjuvant endocrine therapy is the real or perceived side effects. This factor decreases persistance and compliance with therapy.
A new possible approach to adjuvant therapy would be to leverage current combinations of mTor inhibitors or CDK4/6 inhibitors with AIs used successfully for the treatment of AI resistant recurrances. Responsiveness in the treatment of MBC is a good predictor of efficasy as an adjuvant therapy. However, current enormous cost of both mTor inhibitors and CDK4/6 inhibitors, a lack of predictive test for efficasy, and in the case of CDK4/6 inhibitors, up to 50% grade 3 and 4 neutropenia (discussed in reference 13) will together have a major effect on noncompliance during long-term adjuvant therapy. Another path could be taken.
With our aging population, there will be a 50% increase in ER postive breast cancer over the next 30 years. The incidence of ER positive breast cancer is age related. Any oral antihormone therapy, destined to replace AIs as a long-term adjuvant therapy, should provide documented health benefits for the patients and be side effect free to maintain compliance. Future new long-term medicines to treat breast cancer, must now address healthcare issues for elderly women.
Medicinal chemists have already devised a SERM, that can reduce the incidence of osteoporotic fractures, reduce the incidence of breast cancer, reduce coronary heart disease, strokes and endometrial cancer. Perhaps it is time to return to first principles and develop the ideal SERM, but with an acrylic acid side chain that programs the ER complex for destruction. The contortion produced by an acrylic acid SERM ER complex(16), will destroy the over production of tumor ER caused by current AI adjuvant therapy or by ER gene amplification. As a result, no mutated ER would occur (approximately 25% in AI recurrences) so there would be a 25% decrease in recurrences with a new SERM compared to AIs during adjuvant therapy. This would be a wise public health strategy proven health benefits would enhance compliance. Savings for healthcare systems globally would be immense in prevention of diseases associated with menopause, as well as maintaining more patients disease free during longterm adjuvant breast cancer treatment.
Acknowledgments
Funding acknowledgment: This work was supported by the University of Texas MD Anderson Cancer Center support grant to Peter Pisters CA 016672, the benefactors of the Dallas/Ft Worth Living Legend Chair of Cancer Research, and the George and Barbara Bush Endowment for Innovative Cancer Research.
Footnotes
Conflict of interest statement: None to report
References
- 1.Gong CME, Tsoi H, Lee TKW, Paul L, Mak Sai-Ting, et al. BQ323636.1, a novel splice variant to NCOR2, as a predictor for tamoxifen resistant breast cancer. Clin Cancer Res. 2018 doi: 10.1158/1078-0432.CCR-17-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton EPPM, Armstrong AC, Baird RD, Jhaveri K, Hoch M, et al. A first in human study of the new oral selective estrogen receptor degrader AZD9496 for ER+/HER2− advanced breast cancer. Clin Cancer Res. 2018 doi: 10.1158/1078-0432.CCR-17-3102. [DOI] [PubMed] [Google Scholar]
- 3.Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003;9(6):1980–9. [PubMed] [Google Scholar]
- 4.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–16. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan VC. Tamoxifen: catalyst for the change to targeted therapy. Eur J Cancer. 2008;44(1):30–8. doi: 10.1016/j.ejca.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res. 1988;48(4):812–5. [PubMed] [Google Scholar]
- 7.Howell ADD, Anderson H, Redford J. Response after withdrawal of tamoxifen and progestogens in advanced breast cancer. Annals of Oncology. 1992;3(8):611–7. doi: 10.1093/oxfordjournals.annonc.a058286. [DOI] [PubMed] [Google Scholar]
- 8.Fan P, Agboke FA, Cunliffe HE, Ramos P, Jordan VC. A molecular model for the mechanism of acquired tamoxifen resistance in breast cancer. Eur J Cancer. 2014;50(16):2866–76. doi: 10.1016/j.ejca.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottardis MM, Jiang SY, Jeng MH, Jordan VC. Inhibition of tamoxifen-stimulated growth of an MCF-7 tumor variant in athymic mice by novel steroidal antiestrogens. Cancer Res. 1989;49(15):4090–3. [PubMed] [Google Scholar]
- 10.Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20(16):3386–95. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 11.Lonard DM, O’Malley BW. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell. 2007;27(5):691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Weir HM, Bradbury RH, Lawson M, Rabow AA, Buttar D, Callis RJ, et al. AZD9496: An Oral Estrogen Receptor Inhibitor That Blocks the Growth of ER-Positive and ESR1-Mutant Breast Tumors in Preclinical Models. Cancer Res. 2016;76(11):3307–18. doi: 10.1158/0008-5472.CAN-15-2357. [DOI] [PubMed] [Google Scholar]
- 13.Abderrahman BJV. Long-term adjuvant anti-oestrogenic therapy for breast cancer. Clinical Pharmacist. 2016;8(6):180–90. [Google Scholar]
- 14.Willson TM, Henke BR, Momtahen TM, Charifson PS, Batchelor KW, Lubahn DB, et al. 3-[4-(1,2-Diphenylbut-1-enyl)phenyl]acrylic acid: a non-steroidal estrogen with functional selectivity for bone over uterus in rats. J Med Chem. 1994;37(11):1550–2. doi: 10.1021/jm00037a002. [DOI] [PubMed] [Google Scholar]
- 15.Katzenellenbogen JKB, Greene G, Schandarlapaty S, Mayne C. Structural underpinnnings of estrogen receptor mutations in endocrine therapy resistance. Nature Reviews Cancer. 2018 doi: 10.1038/s41568-018-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu YL, Yang X, Ren Z, McDonnell DP, Norris JD, Willson TM, et al. Structural basis for an unexpected mode of SERM-mediated ER antagonism. Mol Cell. 2005;18(4):413–24. doi: 10.1016/j.molcel.2005.04.014. [DOI] [PubMed] [Google Scholar]


