Abstract
Assembly of the mitotic spindle is essential for proper chromosome segregation during mitosis. Maintenance of spindle poles requires precise regulation of kinesin- and dynein-generated forces, and improper regulation of these forces disrupts pole integrity leading to pole fragmentation. The formation and function of the mitotic spindle is regulated by many proteins, including Aurora A kinase, the motor proteins Kif2a and Eg5. Here, we characterize a surprising role for the RhoA GTPase-activating protein, p190RhoGAP, in regulating the mitotic spindle. We show that cells depleted of p190RhoGAP arrest for long periods in mitosis during which cells go through multiple transitions between having bipolar and multipolar spindles. Most of the p190RhoGAP depleted cells finally achieve a stable bipolar attachment and proceed through anaphase. The multipolar spindle phenotype can be rescued by low doses of an Eg5 inhibitor. Moreover, we show that in p190RhoGAP-depleted multipolar cells localize Aurora A to all the poles, but the kinase is only activated at the two centriolar poles. Overall, our data identify an unappreciated connection between p190RhoGAP and the proteins that control spindle poles including Aurora A kinase and Eg5 that is required to prevent or correct spindle pole fragmentation.
Keywords: p190RhoGAP, mitotic spindle, Aurora A, centrosome, Eg5
INTRODUCTION
Rho GTPase-activating Protein 35/RhoGAP P190A/Glucocorticoid receptor DNA-binding factor 1 (p190) is a large RhoGAP that has been implicated in angiogenesis (Mammoto A et al., 2000) and the development of the forebrain, eye and neural tube (Brouns MR et al., 2000). It has also been associated with the tumorigenesis of some cancers including osteosarcomas (Fincham VJ et al., 1999; Zhao J et al., 2014). At the cellular level, it has both cytoplasmic roles in Rho signaling and nuclear roles in transcription (Ridley AJ et al., 1993). P190 regulates the actin cytoskeleton by down regulating Rho activity, and specifically has been shown to promote actin stress fiber disassembly to control many cellular processes including limiting cell migration and dendritic spine morphogenesis (Ridley AJ et al., 1993; Brouns MR et al., 2001; Chang JH et al., 1995). During mitosis p190 regulates cortical rigidity during cell rounding and binds anillin to control the rate of actin contraction during cytokinesis (Manukyan A et al., 2015; Manchinelly SA et al., 2010; Su L et al., 2009; Mikawa M et al., 2008; Su L et al., 2003; Maddox AS et al., 2003).
Chromosome segregation depends upon the assembly of a bipolar mitotic spindle to pull the mitotic chromosomes to opposite poles. The minus ends of spindle microtubules are gathered into two poles by diverse mechanisms including the recruitment of the NuMA protein to the minus ends of the spindle by dynein where NuMA cross-links multiple microtubules (Silk AD et al., 2009; Haren L et al., 2009; Merdes A et al., 1996; Merdes A et al., 2000). Maintenance of spindle poles requires precise regulation of kinesin- and dynein-generated forces, and improper regulation of these forces can disrupt pole integrity leading to pole fragmentation (van Heesbeen RG et al., 2016; van Heesbeen RG et al., 2014). One part of this regulation is through a module of proteins that includes the tetrameric kinesin Eg5, the Microtubule associated protein (MAP) Tpx2 and the Kinase Aurora A (van Heesbeen RG et al., 2016; Garrido G and Vernos I, 2016; Wadsworth P, 2015; Ma N et al., 2010). These proteins are found at the poles, and their proper function is required to prevent pole fragmentation. The regulation of these proteins is poorly understood, but it is clear that these proteins all directly regulate each other (Tsai MY et al., 2003). Aurora A and Tpx2 both inhibit Eg5 microtubule sliding activity (Balchand SK et al., 2015). Tpx2 also directly binds and activates Aurora A by inhibiting T-loop dephosphorylation by PP1 (Zorba A et al., 2014; Bayliss R et al., 2003).
During our previous characterization of the role of p190 in cytokinesis we noted that cells arrest for long periods in mitosis (Manukyan A et al., 2015). Here we characterize the role of p190 in regulating the mitotic spindle that generates this arrest. Specifically, we find that cells depleted of p190 have either longer bipolar spindles or generate multipolar spindles. These phenotypes arise during the first mitosis after depletion of the protein and therefore are not indirect consequences of failure of cytokinesis during a previous division cycle. We find a large number of multipolar spindles in p190 depleted cells and demonstrate that the multipolar spindles seen after p190 depletion are generated by fragmentation of poles rather than entering mitosis with extra centrioles. Time-lapse imaging of p190 depleted cells expressing histone H2b-GFP demonstrate that these cells go through multiple rounds of metaphase alignment followed by loss of alignment. Together these data suggest that the cells are dynamically transitioning between bipolar and multipolar spindles. Eventually most of the cells obtain a stable enough bipolar spindle to traverse into anaphase, demonstrating that most of the mitotic machinery remains intact. The bipolar spindles that we see are longer and can be “wavy” which is a phenotype previously reported for Kif2a manipulation in Xenopus extracts (Geatz and Kapoor, 2004). To gain insight into how p190 regulates spindle poles we analyzed p190 immunoprecipitate by mass spectrometry analysis and identified an interaction with the Eg5 kinesin motor, which regulates pole forces (Shimamoto et al., 2011). Our data suggest that p190 regulates a previously unappreciated step in spindle pole maintenance. Specifically, p190 is required to activate Aurora A at acentrosomal poles, while Aurora A activity at centrosomal poles is unaffected.
MATERIALS AND METHODS
Cell culture and cell synchronization
HeLa cells and RPE cells were obtained from American Tissue Culture Collection (Manassas, VA) and maintained by serial passage in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Carlsbad, CA) containing 10% fetal calf serum (Gibco) and 1% penicillin/streptomycin (Gibco) at 37°C in a 5% CO2, humidified environment. Cells were synchronized by treatment with 2mM thymidine for 24 hours, transfected with p190 siRNA and released into fresh media for 12 hrs. Cells were arrested again in 2mM thymidine, transfected with siRNA a second time for 24 hrs, and released for 8-10 hrs. After release from thymidine, monastrol was added at the indicated concentrations for an additional 1 hr, at which time cells were fixed for immunofluorescence (IF) analysis.
siRNA transfection
Human p190RhoGAP-A was silenced using double-stranded p190 RNAi oligos custom made by Dharmacon that targeted a unique sequence at the N-terminus of the protein. The sequence of the double stranded oligo was 5′ AAG AUG CAC AUU GUG GAG CAG 3′. Glass coverslips were coated with 1mg/ml poly-L-lysine (Sigma), and cells synchronized by thymidine as mention above were treated with p190 siRNA oligos (50 pmol) twice by using the Lipofectamine RNAiMax reagent (Invitrogen), as per the manufacturer’s instructions.
Generation of HeLa Tet-on p190-inducible cell lines and knockdown rescue experiments
The Tet-on expression system obtained from CLONTECH Laboratories, Inc. was used according to the manufacturer’s directions. pcDNA5/FRT/TO-p190, pcDNA5/FRT/TO-p190 (R1283A), expression vectors and the Flp recombinase vector, pOG44, were co-transfected into Flp-In™ HeLa T-REx cells using Lipofectamine 2000 (Invitrogen). Cells were selected in the presence of 0.2 mg/ml Hygromycin B and screened for p190 expression as follows: Dox-inducible HeLa cell lines were plated at 30% density and after 16 hrs, cells were transfected with 75 nM p190 siRNA. 1 μg/ml doxycycline was used to induce expression of full-length p190 or the GAP mutant p190 (R1283A) of p190. Culture medium was changed after 24 hrs, and the cells were allowed to incubate for 48 hrs post-transfection in the presence of 1 μg/ml doxycycline.
Live-cell imaging
To perform live-cell imaging, HeLa cells were plated onto poly-L-lysine coated 1.5 Borosilicate two-well chambered coverglass dishes (LabTex), and images were acquired using a 20X dry objective lens (NA 0.75; Olympus) on a deconvolution microscope (DeltaVision). Single-plane multipoint acquisition were captured on a CoolSNAP HG2 camera (Photometric) every 5 min within a 37°C chamber. Images of cells treated with control or p190 siRNAs were collected with identical exposure times and scaled equally. The acquisition software used was SoftWoRX from Applied Precision.
Cell fixation, Immunofluorescence Microscopy and Quantification
HeLa cells were grown on poly-L-lysine-coated coverslips, depleted of p190, and synchronized as described above for 48 hours. Cells were fixed ether for 5 min with methanol or for 20 min with 4% paraformaldehyde, 1X PHEM (60mM PIPES, 25mM HEPES, 10mM EGTA, 4mM MgCl2, pH-6.9), and then permeabilized for 5 min with 0.2% Triton X-100. After fixation cells were blocked with 20% goat or donkey serum at room temperature for 1 hr and incubated with appropriate primary antibodies: FITC-conjugated anti- α -Tubulin DM1α (mouse, Sigma-Aldrich), γ-Tubulin (rabbit, Abcam), anti-Aurora A (mouse, Abcam), anti-phospho-T288 Aurora A (rabbit, Abcam), anti-centrin2 (mouse, Milipore), anti-Eg5 (rabbit, Abcam), anti-centromere antibodies (ACA, human, Antibodies Inc.), anti-TPX2 (rabbit, Novus) and anti-pKif2A (rabbit, polyclonal antibody obtained from P.T. Stukenberg laboratory, University of Virginia, Charlottesville, VA) for 1 hr at room temperature. Cells were washed with PBS and incubated with FITC and Alexa Fluor 594 conjugated anti-mouse and anti-rabbit secondary antibodies respectively for 1 hr. (Invitrogen). Coverslips were mounted onto glass microscope slides using ProLong® Gold antifade reagent with DAPI as the mounting medium. Cells were analyzed on a Zeiss Axiovert 200 wide-field fluorescence microscope, fitted with a confocal scanner using a krypton/argon laser (Perkin Elmer), Hamamatsu EMCCD camera, a NanoScanZ motor (Prior) and a 63X oil Plan-Apochromat objective. An acousto- optic tunable filter (AOTF) was used for detection of light at 488, 568 and 647 nm. Photographs were taken as z-series with 0.4 μm Z- steps. Exposure times were consistent for each channel throughout individual experiments, and no images were altered after capture. Quantification was performed using Volocity®5.5 (Perkin Elmer). The areas (region of interest-ROI) were outlined using lasso tool (mask) to determine the average pixel intensity per square micron, which was then averaged per cell. Values were corrected for background by subtracting the average pixel intensity per square micron measured using the same mask. Fifteen-thirty cells per treatment were analyzed for each experiment.
Immunoprecipitation and Western blotting
To generate cell lysates for IP and Western blotting, cells were collected and spin down at 1500 rpm. Pellet were washed with PBS (Invitrogen) and resuspended in lyses buffer (50 mM Tris-HCl pH = 7.6, 150 mM NaCl, 5 mM MgCl2, 0.5% Triton X-100). Lysates were sonicated and incubated with 5 μg of p190 antibody (mouse, BD Transduction laboratories) overnight. After primary antibody incubation, immunocomplexes were precipitated by using protein-G–agarose (Invitrogen) and separated by centrifugation at 2300 × g for 1 min. After washes with cold PBS, SDS sample buffer was added to each sample, boiled for 5 minutes, and subjected to western blotting analysis.
RESULTS
p190RhoGAP deficient cells arrest in mitosis
To investigate the role of p190RhoGAP in mitosis we depleted the protein in HeLa cells using p190-specific small interfering RNAs (siRNA) and followed individual cell behavior by time-lapse microscopy using DIC imaging. For each cell, we quantified the cell fate and the time spent in mitosis, which was defined as the time when cells first round up until they reentered interphase or died. The majority of p190-depleted cells delay in mitosis compared to control treated cells independent of their cell fate (Fig. 1, a and b, Supplementary movies 1–4). We noted four independent cell fates triggered by p190 depletion. First, cells were able to exit mitosis and complete cell division after a prolonged mitotic arrest. Second, cells eventually exit mitosis but died right after division. Third, cells died during the mitotic arrest. Fourth, cells segregated their chromosomes but failed cytokinesis as previously reported (Manukyan A et al., 2015)
Fig. 1. Mitotic fates of HeLa cells depleted of p190.
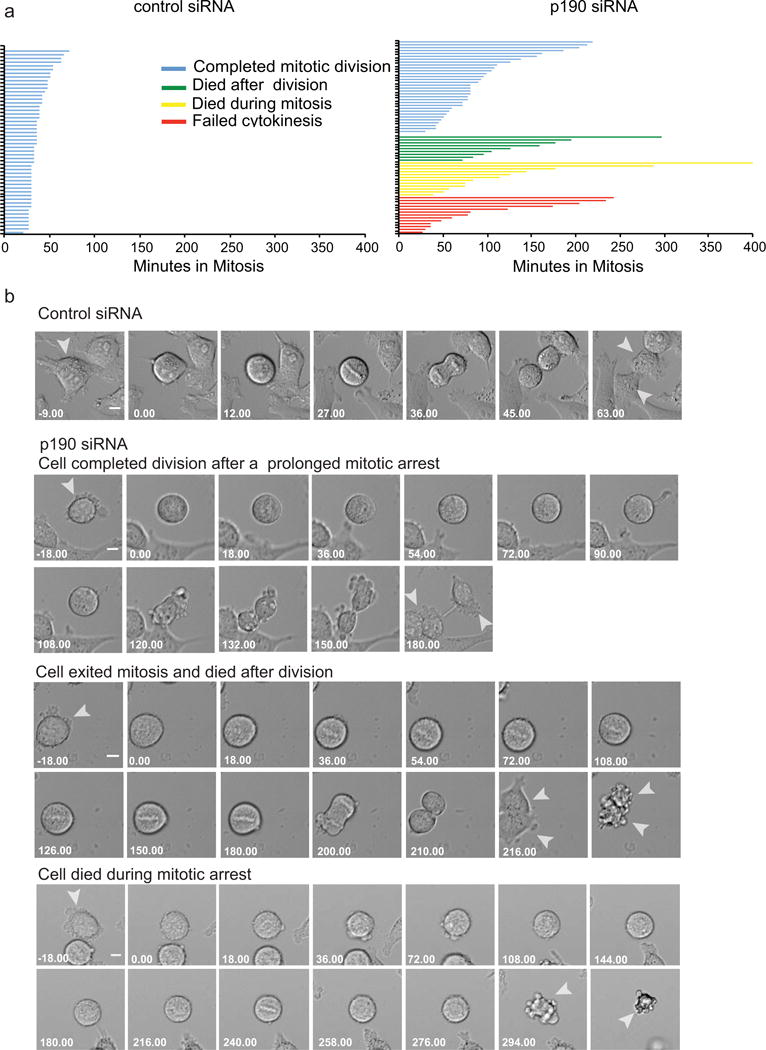
a. Cell fates of HeLa cells as measured by time-lapse imaging after transfection with either control or p190 siRNA. The length of each line represents the time a single cell spent in mitosis, calculated as the time between cell rounding until it re-entered interphase. The color of the line represents the four cell fates triggered by p190 depletion as indicated in the key. b. Representative time-lapse images of the cell fates quantified in the Top panel represent control-siRNA-treated cells. The next three panels show p190- siRNA-treated cells that represent the three cell fates described in the text. Failure to cytokinesis was not shown since it was characterized in our previous paper [Manukyan A et al., 2015]. Arrows indicate single cell divisions after treatment with either siRNA. Time is shown in min. Scale bars-20 μm.
p190RhoGAP is required for bipolar spindle formation and this activity is independent of its GAP activity
We further characterized the mitotic defect caused by p190 depletion by immunofluorescence to visualize the mitotic spindle. To ensure that we visualized the first mitosis after depletion of the protein, cells were double synchronized in S-phase with thymidine, during the treatment with the p190-specific siRNA and then released from thymidine for 10-12 hours. Cells were fixed and stained with tubulin and anti-centromere antibody (ACA) and analyzed by confocal microscopy. p190 depleted cells arrested in mitosis with either multiple poles or with bipoles that had an elongated pole to pole axis (Fig. 2a). Quantification shows that ~30%-50% of p190-depleted cells had multipolar spindles compared to 5% of control siRNA-treated cells (n>200) (Fig. 2b, 3a). The bipolar spindles were 3-5 micron longer than controls in p190-depleted cells (Fig. 2c). Similar phenotypes were seen after the depletion of p190 from nontransformed RPE cells (Fig. 2b and 2c). The efficiency of depletion of p190 after 48 hours siRNA treatment is shown in Fig. 3a.
Fig. 2. p190RhoGAP regulates bipolar spindle formation and spindle length.
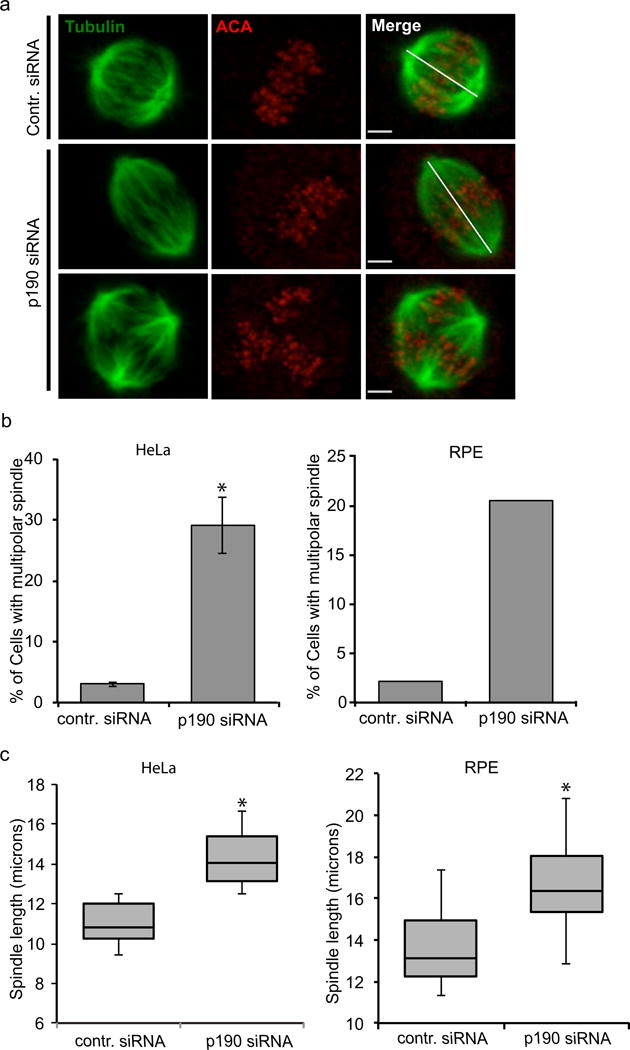
a. Confocal images of control- and p190-siRNA-treated HeLa cells co-stained with alpha tubulin (green) and anti-centromere antibody (ACA) (red). Top panel images represent control-siRNA-treated cell; middle panel images represent p190-depleted cells with longer bipolar spindles; bottom panel images represent p190-depleted cells with multipolar spindles. White lines show how spindle length was measured for control- and p190-siRNA-treated cells. Images shown are representative of n>50. Scale bars represent 2.1 μm. b. Quantification of cells with multipolar spindles stained with tubulin and anti-centromere antibody (n ≥ 200). Results are mean ± s.d from three independent experiments. *P< 0.05 (T-test) as compared to control siRNA-treated cells. c. p190 depletion causes increase of spindle length in HeLa and RPE cells. Spindle lengths were measured in p190-depleted HeLa and RPE cells stained with tubulin and anticentromere antibody. Box-and-whisker plots show median, 25th and 75th percentiles (box) and 5th and 95th percentiles (whiskers) (n ≥ 50). *P< 0.05 (T-test) as compared to control-siRNA-treated cells.
Fig. 3. p190RhoGAP regulates bipolar spindle formation independent of its GAP activity.
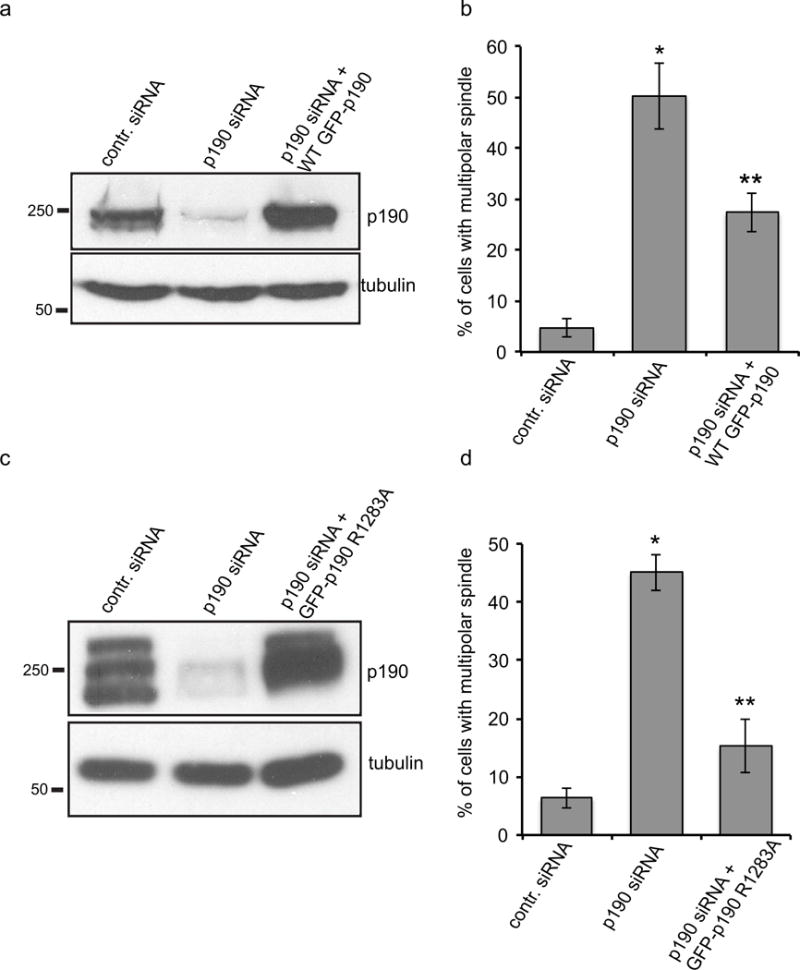
a. Immunoblot to compare endogenous p190 levels to those induced after knockdown of the endogenous p190 levels and expression of the transgene by doxycycline. b. Quantification of multipolar spindle cells after depletion of endogenous p190 and replacement with doxycycline inducible exogenous wild type (n≥200). Results are mean ±s.d from three independent experiments. *P<0.005 (T-test) for both p190-siRNA-treated cells compared to control, and p190-siRNA-treated cells compared to cells rescued by wild-type p190. c. Immunoblot to compare endogenous p190 levels to those induced after knockdown of the endogenous p190 levels and expression of the p190 Gap mutant (R1283A) transgene by doxycycline. d. Quantification of multipolar spindle cells after depletion of endogenous p190 and replacement with the doxycycline-inducible GAP mutant of p190. 48 hrs post-transfection and doxycycline treatment, cells were fixed and stained with tubulin and ACA and scored blindly for multipolar spindle cells (n≥200). Results are mean ± s.d from three independent experiments. *P<0.005 for both p190-siRNA-treated cells compared to control, and p190-siRNA-treated cells compared to cells rescued by p190 GAP mutant (p190 R1283A) compared to control.
To exclude off-target siRNA effects we rescued the phenotype using a previously generated cell line that expresses a siRNA-resistant, doxycycline-inducible transgene of GFP-tagged p190 (Manukyan A et al., 2015). The transgene is expressed at levels similar to the endogenous protein in non-depleted control cells (Fig. 3a). Almost 2/3rds of cells exhibiting a multipolar spindle phenotype could be rescued by replacement of endogenous p190 with doxycycline-inducible exogenous wild type protein (Fig. 3b). Thus, our data suggest that p190 is required for bipolar spindle formation.
Next we examined whether the RhoGAP activity of p190 played a role in bipolar spindle formation. We generated a doxycycline-inducible stable cell line to replace the endogenous p190 with a siRNA-resistant p190 (R1283A) point mutant that is defective in GAP activity. Figure 3c shows that the expression level of the mutant p190 is similar to levels of endogenous protein in control cells. Replacement of endogenous p190 with doxycycline-inducible GAP mutant form of p190 significantly rescued the number of cells exhibiting a multipolar spindle phenotype (Fig. 3d). This result suggests that p190 regulation of bipolar spindle formation is independent of p190’s GAP activity.
p190 depletion results in centrosome fragmentation
Mitotic cells most often become multipolar because they either amplify their centrosomes or because pole forces are not properly controlled, leading to pole fragmentation. p190 depleted HeLa cells were stained with a centriole marker (centrin-2) and tubulin antibodies to determine if they had amplified centrioles. The majority of multipolar cells contained two centrin-containing poles and an extra acentriolar pole(s) (Fig. 4a, c), which supports the loss of spindle pole integrity rather than centrosome amplification. We also stained cells for a component of the pericentriolar material (γ-tubulin), which was found at each of the poles, while centrin was restrained to two poles (Fig. 4b).
Fig. 4. p190 depletion results in pericentriolar, but not centriolar, fragmentation.
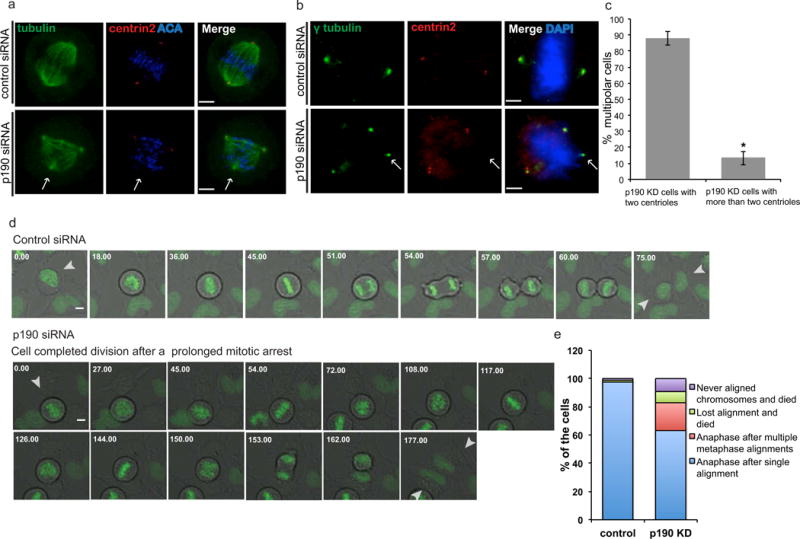
a. Confocal images of control- and p190-depleted cells co-stained with α-tubulin and a centriole marker (centrin2). Arrows indicate extra spindle poles. Images shown are representative of n>20. Scale bars represent 4 μm b. Confocal images of control- and p190-depleted cells co-stained with centrin2 and pericentriolar material component (PCM) γ-tubulin. Arrow indicates the pole that did not stain with centrin2, suggesting that multipolarity generated by loss of p190 is the result of PCM fragmentation. Images shown are representative of n>20. Scale bars represent 4 μm. c. Percentage of the cells with two centrioles are graphed versus percentage of the cells that contained more than two centrioles (n≥50, means of 2 experiments). d. Representative time-lapse images of the control and p190 depleted cells with GFP tagged H2B chromatin marker. Top panel represent control-siRNA-treated cells. Bottom panels show p190-siRNA-treated cells. Arrows indicate single cell divisions after treatment with either siRNA. Time is shown in min. Scale bars 20 μm. e. Quantification of time-lapse images of control and p190 siRNA treated HeLa cells according to chromosome alignment (p=1.8e−06, Fisher’s exact test).
Time-lapse movies of GFP-tubulin in p190 depleted cells were difficult to interpret (not shown) so we visualized the movements of chromosomes on the mitotic spindle by expressing histone H2b-GFP (Fig. 4d, Supplementary movies 5–6). Surprisingly a significant number of p190 depleted cells went through rounds of aligning chromosomes and then the alignment was lost (p=1.8e−06, Fisher’s exact test). Most of the time these cells finally obtained a bipolar configuration and entered anaphase. In addition, a small number of cells died without aligning chromosomes or after losing chromosome alignment (Fig 4e). Together these data demonstrate that the p190 is required for stable bipolar spindles and suggest that multipolar spindles are transiently formed by pole fragmentation.
Spindle bipolarity is rescued by inhibition of the kinesin Eg5
We performed an unbiased purification of p190RhoGAP, to determine if p190 interacts with any proteins involved in bipolar spindle formation. p190 was immunoprecipitated from cells synchronized in mitosis by a double thymidine block and precipitated proteins were analyzed by mass spectrometry. Interestingly, peptides from the kinesin-5 family motor protein Eg5 were found in the p190 immunoprecipitate that were not identified in a control IgG purification. Eg5 is a plus-end-directed homotetrameric kinesin, which cross-links antiparallel microtubules in the mitotic spindle and is required for spindle bipolarity (van den Wildenberg SM et al., 2008; Sawin KE and Mitchison TJ, 1995; Kapitein LC et al., 2005). Eg5 inhibition by either RNA interference or small molecule inhibitor such as monastrol results in monopolar spindle formation and mitotic arrest (Kapoor TM et al., 2000; Mayer TU et al., 1999). Moreover, it also has been shown that Eg5 overexpression causes the formation of multipolar spindles by pole fragmentation (Castillo A et al., 2007; Liu M et al., 2010). Thus we hypothesized p190 inhibits Eg5 activity. In theory, p190 could regulate Eg5 expression, localization or activity, so each possibility was systematically tested. First, p190 was depleted in HeLa cells by siRNA, and the subcellular localization of Eg5 was assessed by confocal microscopy. Eg5 localized to spindle microtubules and was enriched near spindle poles of both control and p190-depleted cells (Fig. 5a). We measured the overall intensity of total Eg5 in each cell by immunofluorescence and western blot (Fig. 5b (n>20), 5c). Neither was significantly changed after p190-depletion as compared to controls. We next tested whether the multipolar spindles were generated by excessive motor activity of Eg5. HeLa cells depleted of p190 were synchronized using a double thymidine block. After release from thymidine for 10 hours, HeLa cells were treated for one and one-half hours with different doses of the Eg5 ATPase inhibitor, monastrol, and multipolar cells were scored. Interestingly, we found that low doses of monastrol partially rescued the multipolar spindle phenotype caused by p190 depletion (Fig. 5d). The doses that rescued the activity were lower than those that generated monopolar spindles. The simplest interpretation of these data is that p190 regulates Eg5 activity; although it is possible that monastrol rescues bipolarity by a more complicated mechanism. For example, the depletion of p190 leads to unbalanced spindle forces, which are rescued by partial inhibition of Eg5.
Fig. 5. p190 controls Eg5activity.
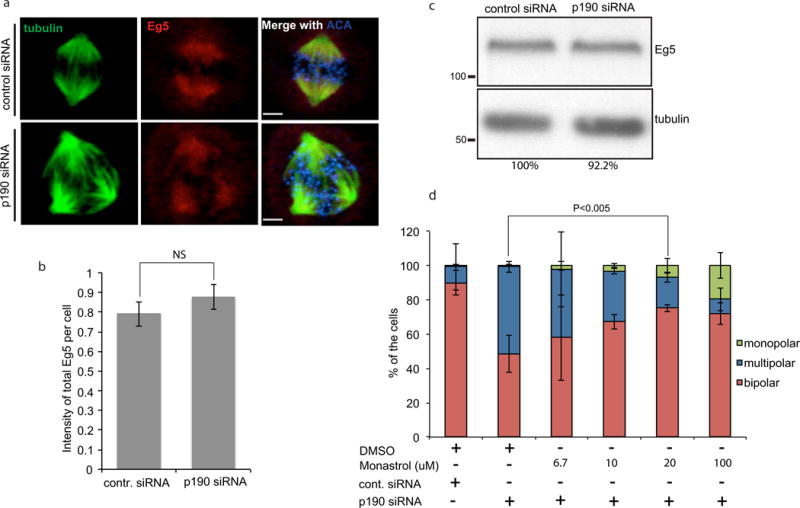
a. Eg5 localized properly in p190-depleted cells. Confocal images of control- and p190-siRNA treated cells that were fixed and stained with α-tubulin, ACA and anti-Eg5 antibody. Images shown are representative of n>20 bars represent 4 μm. b. Quantification of Eg5 intensity at poles in HeLa cells (p =NS). c. Immunoblot of endogenous Eg5 in control and p190 KD cells. Eg5:tubulin ratio were quantified and amount were reported by percentage. d. Multipolar spindles induced by p190 depletion are rescued by inhibition of Eg5 activity. p190-depleted and double thymidine-blocked cells were released from thymidine for 10 hours and treated with DMSO or 6.7μM, 10μM, 20μM or 100μM monastrol for one and a half hrs. Cells were fixed and stained with tubulin and ACA antibodies, and monopolar, bipolar and multipolar cells were quantified. Results are mean ± s.d from three independent experiments. The rescue of bipolar spindles by 20 uM monastrol is statistically significant (*P<0.005).
p190RhoGAP does not affect TPX2 localization, but it is required to activate Aurora A on acentriolar poles
We tested whether proteins known to function with Eg5 (Tpx2 and Aurora A) are also under p190 control. Inhibition of Aurora A kinase also generates multipolar spindles by spindle pole fragmentation (Asteriti IA et al., 2011). Aurora A interacts with and phosphorylates many proteins including Tpx2 and Eg5 (Tsai MY et al., 2003; Giet R and Prigent C, 1999; Giet R et al., 1999; Kufer TA et al., 2003; Kufer TA et al., 2002). We asked whether Aurora A localized properly to poles and whether it was active at each pole in p190-depleted cells by immunostaining with antibodies to Aurora A and its activation loop (pT288). Interestingly, we found that Aurora A was localized to all the poles; however, the T-loop staining was only at two poles of a multipolar spindle (Fig. 6a). This suggests that Aurora A is not activated in that extra pole. Similarly, we found that the phospho-antibody against an Aurora A site on the kinesin, Kif2a, stained only two poles of a multipolar spindle (Fig. 6b). To determine if this phenotype was caused by p190 depletion we asked if the poles of multipolar cells of control cells (that spontaneously arise ~5% of the time) also had lower Aurora A activity. We quantified the intensity of total Aurora A and phospho-T288 Aurora A and plotted the ratio at each pole as a measure of the activation of Aurora A at each pole. In addition, we quantified this ratio in control Hela cells that were multipolar and segregated these from the bipolar control cells (Fig. 6c). We normalized all ratios to the average of bipolar control cells. In control bipolar cells the activation of Aurora A was very uniform, as the distribution was tightly clustered. Aurora A activity was more widely distributed on the multipolar spindles of control cells, but the ratios showed a single distribution. In contrast, the multipolar spindles in p190-depleted cells had a bimodal distribution. One peak was similar to the control bipolar spindles and a second peak that was significantly lower (Fig. 6c).
Fig. 6. p190 is required to activate Aurora A kinase on acentriolar poles.
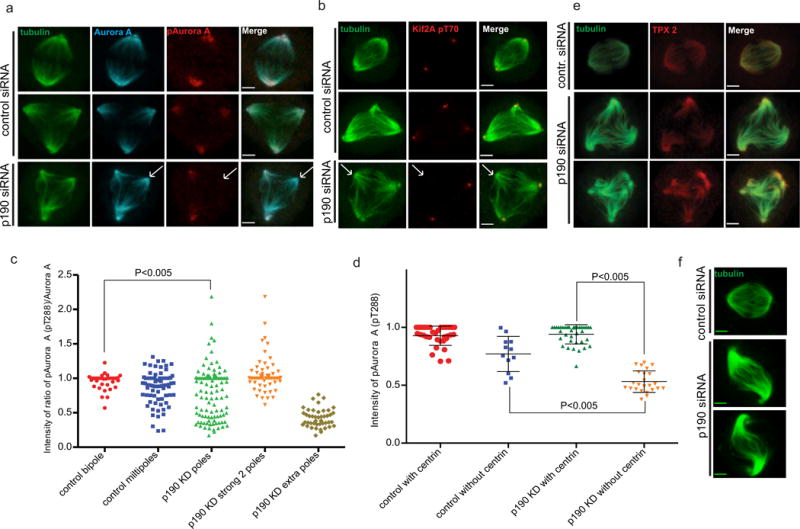
a. Aurora A is properly localized to all poles but it is not active at all poles. Confocal images of control- and p190-depleted cells fixed and co-stained with α-tubulin, Aurora A and T-loop phosphorylation for Aurora A (pT288) antibodies. First two panels represent examples of bipolar and multipolar HeLa cells treated with control siRNA; the third panel represents a p190-depleted multipolar cell. Arrows indicate an extra spindle pole in a p190-depleted cell. Images shown are representative of n>20. Scale bars represent 3 μm. b. Aurora A activity as measured with a phosphoantibody against an Aurora site on Kif2a is missing from poles that lack centrosomes. Arrows indicate extra spindle poles in p190-depleted cells. Images shown are representative of n>20. Scale bars represent 3 μm. c. Quantification of Aurora A activation in spindle poles measured as a ratio of T-loop divided by total Aurora A. Each dot in the plot represents a paired measurement of T-loop and Aurora A at a single centrosome. Ratio’s are not statistically different between control bipoles and multipoles but they are both different from p190-depleted poles P<0.005 (T test). To demonstrate that the bimodal distribution of Aurora activation is generated by differences between poles in the same cell we replotted the data in the third lane. The degree of Aurora A activation in the highest two poles is different than the levels in the additional pole in the same cell. d. p190 activates Aurora A at acentriolar poles. Control- and p190-depleted cells were fixed and co-stained with α-tubulin, centrin 2 and phospho-Aurora A pT288 antibodies. Intensity of phospho –T288 Aurora A was measured in each pole with or without a centriole in both control- and p190-depleted cells and plotted as a dot plot P<0.005 (ANOVA). e. Tpx2 levels at poles are not affected by p190 knockdown. Images shown are representative of n>20. Scale bars represent 3 μm. f. Confocal images of tubulin stained control and p190-depleted cells with wavy” bipolar spindles. P<0.005
This bimodal distribution could be formed by a mixture of cells that have either highly active or low to no active Aurora A on all poles. Alternatively, p190 depleted cells could have both high and low Aurora A activity poles on the same spindle. To distinguish between these models, we grouped the data from the p190-depleted cells into two plots: the first contained the values from the two poles with the highest ratios of Aurora A (Fig. 6c, p190 KD strong 2 poles); the second contained the values from the remaining poles in the cell (Fig. 6c, p190 KD extra poles). The data segregated into high and low groups, arguing that the bimodal distribution of Aurora A activities found in the multipolar spindles of p190-depleted cells arises from having two poles with high Aurora A activity, while the rest of the poles have low or inactive Aurora A. To determine whether the acentiolar poles had the low or inactive Aurora A, we co-stained control and p190 depleted cells with pT288, centrin 2 and tubulin antibodies. We identified a set of multipolar control cells with two centriolar poles and extra acentriolar poles and compared these to p190 depleted multipolar cells (Fig. 6d). The pT288 staining at the poles with centrioles was similar in both p190 depleted and control cells. In contrast, the pT288 staining of the acentriolar poles in the p190-depleted cells was significantly lower than that in control cells. We therefore concluded that a function of p190 is to activate Aurora A at acentriolar poles.
Tpx2 interacts with Eg5 and inhibits its activity (Garrett S et al., 2002; Koffa MD et al., 2006; Eckerdt F et al., 2008). Ma et al. (2010 (2011) showed that Tpx2 reduced the rate of Eg5-dependent microtubule gliding and inhibited its microtubule sliding activity. Defects in Tpx2 cause multipolar spindle formation due to PCM fragmentation or premature centriole disengagement (Garrett S et al., 2002; Ciciarello M et al., 2004). However, we could not detect significant changes to Tpx2 localization to either pole or pole-radiated microtubules in p190-depleted cells (Fig. 6 e). Figure 6b shows that Kif2a is also misregulated at acentriolar poles. Aurora kinases regulate the localization and activity of the Kif2 family of microtubule depolymerases. We often see “wavy” bipolar spindles (Figure 6f) and elongated spindles (Fig 2a) after p190 depletion, which has been reported for loss of Kif2a activity in Xenopus extracts (Gatez and Kapoor 2004). These data suggest that p190 regulates the Kif2 family of kinesins, most likely through misregulation of Aurora A kinase.
DISCUSSION
p190 is a central mediator of actin-mediated force generation important for a number of cellular and tissue-wide processes. We have identified a new, surprising role for this protein in regulating the mitotic spindle. Our time-lapse imaging demonstrated that depletion of p190 generates a prolonged mitotic arrest. This arrest correlated with longer bipolar mitotic spindles and a propensity to generate multipolar spindles as assayed in fixed cells. Time lapse imaging of mitotic chromosomes showed that they went through rounds of alignment to the metaphase plate followed by loss of alignment, which to our knowledge is a new mitotic phenotype. We suggest that cells lose their alignment when they form multipolar spindles. Unfortunately, our attempts to directly image spindles of p190 depleted cells with GFP-tubulin were unsuccessful. We could see control spindles well, but p190 depleted cells never came into clear focus. We think that this is because those spindles of p190 depleted cells were very dynamic. Together we feel that the simplest interpretation of our data is that p190 depleted cells form bipolar spindles that align chromosomes and these can either enter anaphase or one of the poles splits apart to form a transient multipolar spindle.
Our data suggest that the primary defect to the mitotic spindle seen after p190 depletion is an imbalance of the spindle pole forces for five reasons. First, p190-depleted cells have longer mitotic spindles. Second, these longer spindles often contain wavy bundles of microtubules suggesting that some microtubules are longer than others, a phenomenon which generates a force imbalance that bends bundles of microtubules. Third, about 30% of the spindles become multipolar. We suggest that the wavy spindles are intermediates that eventually break off to form multipolar spindles. Fourth, we see misregulation of Aurora A at acentriolar spindle poles. Fifth, we measure poor Aurora phosphorylation of Kif2a at multipoles, which is the major protein that regulates the depolymerization of microtubules at spindle poles.
Actin is emerging as a critical regulator of spindle bipolarity. Therefore, we were surprised when we found that a p190 mutated in its RhoGAP activity could rescue the multipolar defect brought about by the depletion of the endogenous protein. p190 is a large protein with 4 FF domains and domains predicted to bind GTP. It is also known to bind other proteins, such as p120RasGAP (Hu KQ and Settleman J, 1997). We note that the RhoGAP activity was required to rescue the cytokinesis defects of p190 demonstrating that the GAP mutant is an excellent separation of function tool to study p190’s role in cytokinesis, in the absence of the spindle pole roles characterized here.
p190 regulates Aurora A in response to imbalance of spindle forces at poles
Because is it experimentally difficult to distinguish subregions of a spindle pole, we focused on the regulation of the poles that do not have centrioles (acentriolar poles) in multipolar spindles and we assumed that the regulation at these poles should provide insight into the regulation that normally happens within a spindle pole. This approach identified a surprising form of Aurora A regulation. Specifically, p190 was required to activate Aurora A only at the poles that don’t have centrioles. Our interpretation is that p190 activates Aurora A in response to an imbalance of forces at a spindle pole. This activation in turn corrects the imbalance to maintain spindle bipolarity. In the absence of p190 these forces persist and eventually split off a group of microtubules into a separate pole. Interestingly, the related kinase Aurora B coordinates the correction of kinetochore-microtubule attachments and may release some attachments while leaving others in the same kinetochore alone. Thus, local control of subregions of a large microtubule structures may be a shared function of the Aurora family of kinases.
An important area of future research will be to determine how p190 controls Aurora A activity. We have found that Aurora A is localized properly to the acentrioloar poles of p190-depleted cells but is not phosphorylated on its T-loop, which argues that it is not active at that pole. We similarly find that an Aurora substrate, Kif2a, is not phosphorylated at acentriolar poles in p190- depleted cells. Aurora A is activated by Tpx2 binding and autophosphorylation, which is thought to be initiated by concentration of the kinase at subcellular structures. However, we see little difference in either the amount of Tpx2 or Aurora A at the acentriolar poles, which suggests that the regulation may be through inactivation of inhibitors of kinase activity such as protein phosphatase-6, which is the major T-loop phosphatase for Aurora A. Our data also suggest an important substrate of p190 regulated Aurora A may be the Kif2a. First, p190 depletion phenocopies the “wavy” and elongated spindle phenotypes seen after manipulating Kif2a in Xenopus extracts. Second, phosphorylated Kif2a is depleted from acentriolar poles.
Supplementary Material
Acknowledgments
This study was supported by NIH R01 GM118798 and the authors would like to thank Anindya Dutta for interim support that was crucial for this work.
Footnotes
Conflict of Interest
All of the authors declare that he/she has no conflict of interest.
Ethical approvals:
There were no animals used in this study.
This article does not contain any studies with human participants performed by any of the authors.
References
- Asteriti IA, Giubettini M, Lavia P, Guarguaglini G. Aurora-A inactivation causes mitotic spindle pole fragmentation by unbalancing microtubule-generated forces. Mol Cancer. 2011;10:131. doi: 10.1186/1476-4598-10-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balchand SK, Mann BJ, Titus J, Ross JL, Wadsworth P. TPX2 Inhibits Eg5 by Interactions with Both Motor and Microtubule. J Biol Chem. 2015;290(28):17367–79. doi: 10.1074/jbc.M114.612903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss R, Sardon T, Vernos I, Conti E. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol Cell. 2003;12(4):851–62. doi: 10.1016/s1097-2765(03)00392-7. [DOI] [PubMed] [Google Scholar]
- Brouns MR, Matheson SF, Hu KQ, Delalle I, Caviness VS, Silver J, Bronson RT, Settleman J. The adhesion signaling molecule p190 RhoGAP is required for morphogenic processes in normal development. Development. 2000;127:4891–903. doi: 10.1242/dev.127.22.4891. [DOI] [PubMed] [Google Scholar]
- Brouns MR, Matheson SF, Settleman J. P190 RhoGAP is the principal Src substrate in brain and regulates axon outgrowth, guidance and fasciculation. Nat Cell Biol. 2001;3(4):361–7. doi: 10.1038/35070042. [DOI] [PubMed] [Google Scholar]
- Fincham VJ, Chudleigh A, Frame MC. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J Cell Sci. 1999;112(Pt 6):947–56. doi: 10.1242/jcs.112.6.947. [DOI] [PubMed] [Google Scholar]
- Castillo A, Morse HC, 3rd, Godfrey VL, Naeem R, Justice MJ. Overexpression of Eg5 causes genomic instability and tumor formation in mice. Cancer Res. 2007;67(21):10138–47. doi: 10.1158/0008-5472.CAN-07-0326. [DOI] [PubMed] [Google Scholar]
- Chang JH, Gill S, Settleman J, Parsons SJ. c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation. J Cell Biol. 1995;130(2):355–68. doi: 10.1083/jcb.130.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciciarello M, Mangiacasale R, Thibier C, Guarguaglini G, Marchetti E, Di Fiore B, Lavia P. Importin beta is transported to spindle poles during mitosis and regulates Ran-dependent spindle assembly factors in mammalian cells. J Cell Sci. 2004;117(Pt 26):6511–22. doi: 10.1242/jcs.01569. [DOI] [PubMed] [Google Scholar]
- Eckerdt F, Eyers PA, Lewellyn AL, Prigent C, Maller JL. Spindle pole regulation by a discrete Eg5-interacting domain in TPX2. Curr Biol. 2008;18(7):519–25. doi: 10.1016/j.cub.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett S, Auer K, Compton DA, Kapoor TM. hTPX2 is required for normal spindle morphology and centrosome integrity during vertebrate cell division. Curr Biol. 2002;12(23):2055–9. doi: 10.1016/s0960-9822(02)01277-0. [DOI] [PubMed] [Google Scholar]
- Garrido G, Vernos I. Non-centrosomal TPX2-Dependent Regulation of the Aurora A Kinase: Functional Implications for Healthy and Pathological Cell Division. Front Oncol. 2016;6:88. doi: 10.3389/fonc.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geatz J, Kapoor TM. Dynein/dynactin regulate metaphase spindle length by targeting depolymerizing activities to spindle poles. J Cell Biol. 2004;166(4):465–471. doi: 10.1083/jcb.200404015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet R, Prigent C. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. J Cell Sci. 1999;112(Pt 21):3591–601. doi: 10.1242/jcs.112.21.3591. [DOI] [PubMed] [Google Scholar]
- Giet R, Uzbekov R, Cubizolles F, Le Guellec K, Prigent C. The Xenopus laevis aurora-related protein kinase pEg2 associates with and phosphorylates the kinesin-related protein XlEg5. J Biol Chem. 1999;274(21):15005–13. doi: 10.1074/jbc.274.21.15005. [DOI] [PubMed] [Google Scholar]
- Haren L, Gnadt N, Wright M, Merdes A. NuMA is required for proper spindle assembly and chromosome alignment in prometaphase. BMC Res Notes. 2009;2:64. doi: 10.1186/1756-0500-2-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu KQ, Settleman J. Tandem SH2 binding sites mediate the RasGAP-RhoGAP interaction: a conformational mechanism for SH3 domain regulation. EMBO. 1997;16(3):473–483. doi: 10.1093/emboj/16.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Peterman EJ, Kwok BH, Kim JH, Kapoor TM, Schmidt CF. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature. 2005;435(7038):114–8. doi: 10.1038/nature03503. [DOI] [PubMed] [Google Scholar]
- Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J Cell Biol. 2000;150(5):975–88. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffa MD, Casanova CM, Santarella R, Köcher T, Wilm M, Mattaj IW. HURP is part of a Ran-dependent complex involved in spindle formation. Curr Biol. 2006;16(8):743–54. doi: 10.1016/j.cub.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Kufer TA, Nigg EA, Sillje HH. Regulation of Aurora-A kinase on the mitotic spindle. Chromosoma. 2003;112(4):159–63. doi: 10.1007/s00412-003-0265-1. [DOI] [PubMed] [Google Scholar]
- Kufer TA, Silljé HH, Körner R, Gruss OJ, Meraldi P, Nigg EA. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J Cell Biol. 2002;158(4):617–23. doi: 10.1083/jcb.200204155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Wang X, Yang Y, Li D, Ren H, Zhu Q, Chen Q, Han S, Hao J, Zhou J. Ectopic expression of the microtubule-dependent motor protein Eg5 promotes pancreatic tumourigenesis. J Pathol. 2010;221:221–8. doi: 10.1002/path.2706. [DOI] [PubMed] [Google Scholar]
- Ma N, Titus J, Gable A, Ross JL, Wadsworth P. TPX2 regulates the localization and activity of Eg5 in the mammalian mitotic spindle. J Cell Biol. 2011;195(1):87–98. doi: 10.1083/jcb.201106149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Tulu US, Ferenz NP, Fagerstrom C, Wilde A, Wadsworth P. Poleward transport of TPX2 in the mammalian mitotic spindle requires dynein, Eg5, and microtubule flux. Mol Biol Cell. 2010;21(6):979–88. doi: 10.1091/mbc.E09-07-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox AS, Burridge K. RhoA is required for cortical retraction and rigidity during mitotic cell rounding. J Cell Biol. 2003;160(2):255–65. doi: 10.1083/jcb.200207130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LE, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457(7233):1103–8. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchinelly SA, Miller JA, Su L, Miyake T, Palmer L, Mikawa M, Parsons SJ. Mitotic down-regulation of p190RhoGAP is required for the successful completion of cytokinesis. J Biol Chem. 2010;285(35):26923–32. doi: 10.1074/jbc.M110.103804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manukyan A, Ludwig K, Sanchez-Manchinelly S, Parsons SJ, Stukenberg PT. A complex of p190RhoGAP-A and anillin modulates RhoA-GTP and the cytokinetic furrow in human cells. J Cell Sci. 2015;128(1):50–60. doi: 10.1242/jcs.151647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286(5441):971–4. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- Merdes A, Heald R, Samejima K, Earnshaw WC, Cleveland DW. Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J Cell Biol. 2000;149(4):851–62. doi: 10.1083/jcb.149.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A, Ramyar K, Vechio JD, Cleveland DW. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87(3):447–58. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Mikawa M, Su L, Parsons SJ. Opposing roles of p190RhoGAP and Ect2 RhoGEF in regulating cytokinesis. Cell Cycle. 2008;7(13):2003–12. doi: 10.4161/cc.7.13.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Self AJ, Kasmi F, Paterson HF, Hall A, Marshall CJ, Ellis C. Rho family GTPase activating proteins p190, bcr and RhoGAP show distinct specificities in vitro and in vivo. EMBO J. 1993;12(13):5151–60. doi: 10.1002/j.1460-2075.1993.tb06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE, Mitchison TJ. Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle. Proc Natl Acad Sci USA. 1995;92(10):4289–93. doi: 10.1073/pnas.92.10.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk AD, Holland AJ, Cleveland DW. Requirements for NuMA in maintenance and establishment of mammalian spindle poles. J Cell Biol. 2009;184(5):677–90. doi: 10.1083/jcb.200810091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto Y, Maeda YT, Ishiwata S, Libchaber AJ, Kapoor TM. Insights into the micromechanical properties of the metaphase spindle. Cell. 2011;145(7):1062–1074. doi: 10.1016/j.cell.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Agati JM, Parsons SJ. p190RhoGAP is cell cycle regulated and affects cytokinesis. J Cell Biol. 2003;163(3):571–82. doi: 10.1083/jcb.200308007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Pertz O, Mikawa M, Hahn K, Parsons SJ. p190RhoGAP negatively regulates Rho activity at the cleavage furrow of mitotic cells. Exp Cell Res. 2009;315(8):1347–59. doi: 10.1016/j.yexcr.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MY, Wiese C, Cao K, Martin O, Donovan P, Ruderman J, Prigent C, Zheng Y. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat Cell Biol. 2003;5(3):242–8. doi: 10.1038/ncb936. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg SM, Tao L, Kapitein LC, Schmidt CF, Scholey JM, Peterman EJ. The homotetrameric kinesin-5 KLP61F preferentially crosslinks microtubules into antiparallel orientations. Curr Biol. 2008;18(23):1860–4. doi: 10.1016/j.cub.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heesbeen RG, Raaijmakers JA, Tanenbaum ME, Halim VA, Lelieveld D, Lieftink C, Heck AJ, Egan DA, Medema RH. Aurora A, MCAK, and Kif18b promote Eg5-independent spindle formation. Chromosoma. 2016 doi: 10.1007/s00412-016-0607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heesbeen RG, Tanenbaum ME, Medema RH. Balanced activity of three mitotic motors is required for bipolar spindle assembly and chromosome segregation. Cell Rep. 2014;8(4):948–56. doi: 10.1016/j.celrep.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Wadsworth P. TPX2. Curr Biol. 2015;25(24):1156–8. doi: 10.1016/j.cub.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Xu H, He M, Wang Z, Wu Y. Rho GTPase-activating protein 35 rs1052667 polymorphism and osteosarcoma risk and prognosis. Biomed Res Int. 2014;396947 doi: 10.1155/2014/396947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorba A, Buosi V, Kutter S, Kern N, Pontiggia F, Cho YJ, Kern D. Molecular mechanism of Aurora A kinase autophosphorylation and its allosteric activation by TPX2. Elife. 2014;3:e02667. doi: 10.7554/eLife.02667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


