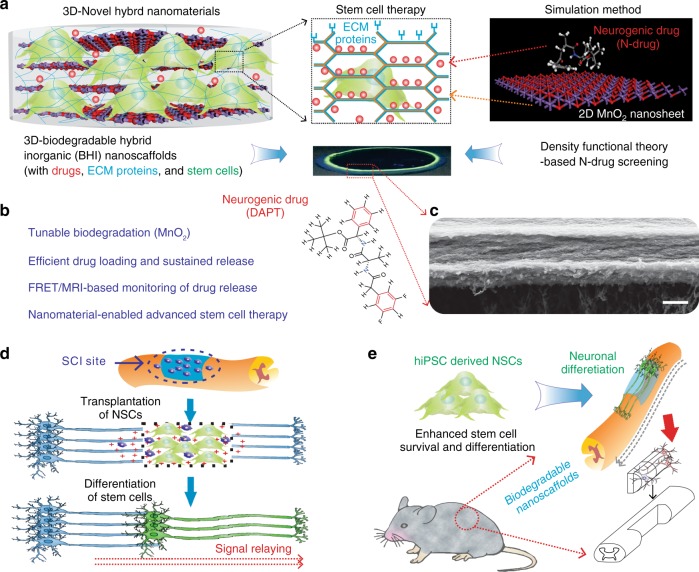
Fig. 1.
BHI nanoscaffolds for advanced stem cell therapy. a To develop an effective method for stem cell transplantation, we synthesized a BHI nanoscaffolds that simultaneously integrate advancements in 3D-hybrid nanomaterials and DFT calculations-based precision drug screening. Cells are labeled with green due to their green fluorescence protein labeling. Laminin proteins are colored in blue. Drugs are represented by red-colored dots. In the simulation scheme, blue colored atoms represent manganese and red colored atoms represent oxygen. b Compared to conventional inorganic scaffolds for stem cell transplantation, our BHI nanoscaffold self-assembled from atomic-thin MnO2 nanosheets, ECM proteins, and therapeutic drugs has unique advantages including: (i) Redox mediated tunable biodegradation; (ii) Efficient drug loading and sustainable release; (iii) FRET/MRI monitorable drug release; (iv) Nanomaterials enabled advanced stem cell transplantation. c A representative SEM (Scanning Electron Microscopy) image of BHI nanoscaffolds. d–e The unique advantages from our innovative BHI nanoscaffold effectively improved the stem cell transplantation under CNS injured microenvironments, which typically have highly inflammatory and inhibitory microenvironments at the injury site. Blue colored and elongated cells indicate host neurons; dark-blue cells with pink nuclei: immune cells such as macrophage; red cross: inflammatory and inhibitory microenvironments. Specifically, the significantly enhancement of stem cell transplantation from BHI nanoscaffold is hypothesized to achieve through an improved cell adhesion and neuronal differentiation. A murine hemisection SCI model was used to evaluate the in vivo survival and differentiation of BHI nanoscaffold-transplanted iPSC-NSCs. Scale bar: 500 nm