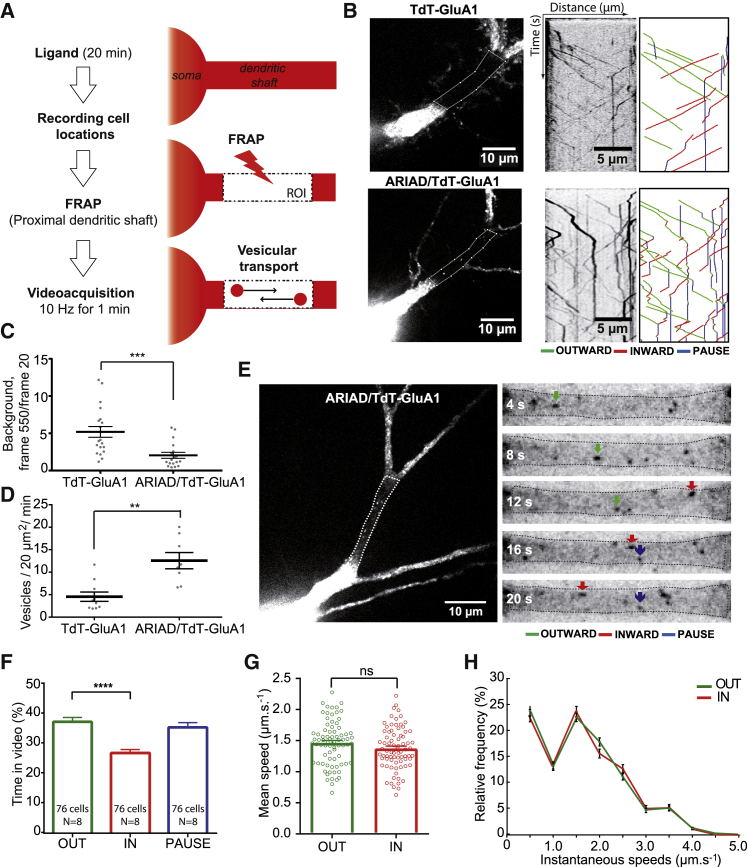
Figure 2.
Characterization of GluA1-Containing AMPAR Intracellular Transport in Basal Condition
(A) Protocol to monitor AMPAR vesicular transport in the proximal neuronal dendritic shaft. After incubation with AL, positions of transfected neurons are registered. GluA1 vesicular transport becomes visible after photo-bleaching (FRAP) of the region of interest (ROI) in the proximal dendrite. Transport is recorded by streaming one image every 100 ms during 1 min.
(B) Images of neurons expressing TdT-GluA1 in pRK5 (top panel) or in ARIAD (bottom panel), with raw and annotated kymographs (right panels). Trajectories are shown with a color code (green for outward movements, red for inward movements, and blue for pausing vesicles). Dotted lines in the crude image delineate the ROI from which kymographs were generated.
(C) Background analyses of the ROI after FRAP of neurons in the two conditions. Results are expressed as the ratio of the background measured with ImageJ at the end of the acquisition (frame 550) over the one measured just after the FRAP (frame 20).
(D) Mean vesicle number passing through a 20 μm2 bleached ROI during 1 min of recording.
(E) Image of neurons expressing ARIAD/TdT-GluA1 (left panel) and the zoomed images of its ROI (right panels) at different time points (t = 4–20 s). Colored arrows indicate outward (green), inward (red), or static (blue) trajectories of GluA1-containing vesicles.
(F) Percentage of time spent in each state by a vesicle containing ARIAD/TdT-GluA1.
(G) Velocities of ARIAD/TdT-GluA1 vesicles.
(H) Mean frequency distribution of instantaneous speeds of mobile GluA1 vesicles (>500 values).
For (C) and (D), mean ± SEM of 2 independent experiments (n = 20/18 cells). For (F) to (H), mean ± SEM of 8 independent experiments (n = 76 cells). See also Figures S2A–S2C and Tables S1 and S2.