Abstract
Faba beans are highly nutritious because of their high protein content: they are a good source of mineral nutrients, vitamins, and numerous bioactive compounds. Equally important is the contribution of faba bean in maintaining the sustainability of agricultural systems, as it is highly efficient in the symbiotic fixation of atmospheric nitrogen. This article provides an overview of factors influencing faba bean yield and quality, and addresses the main biotic and abiotic constraints. It also reviews the factors relating to the availability of genetic material and the agronomic features of faba bean production that contribute to high yield and the improvement of European cropping systems. Emphasis is to the importance of using new high-yielding cultivars that are characterized by a high protein content, low antinutritional compound content, and resistance to biotic and abiotic stresses. New cultivars should combine several of these characteristics if an increased and more stable production of faba bean in specific agroecological zones is to be achieved. Considering that climate change is also gradually affecting many European regions, it is imperative to breed elite cultivars that feature a higher abiotic–biotic stress resistance and nutritional value than currently used cultivars. Improved agronomical practices for faba bean crops, such as crop establishment and plant density, fertilization and irrigation regime, weed, pest and disease management, harvesting time, and harvesting practices are also addressed, since they play a crucial role in both the production and quality of faba bean.
Keywords: landraces, legume, nutritional value, soil fertility, sustainability, weed management, Vicia faba
Introduction
Faba bean (Vicia faba L.) is one of the most globally important legume crops. Its global acreage declined from 3.7 to 2.1 million ha between 1980 and 2014, and yields are highly variable within specific countries (FAO, 2017). Despite the decreasing acreage, however, productivity per area has tended to increase, due to a reduced susceptibility to abiotic and biotic stresses (Link et al., 2010; Sillero et al., 2010; Singh et al., 2012). The global production of faba bean grain in 2014 was 4.1 million tons, which is approximately 21% greater than in 1994 (FAO, 2017). The fresh and dry seeds of faba bean are used for human consumption; they are highly nutritious because they have a high protein content (up to 35% in dry seeds), and are a good source of many nutrients, such as K, Ca, Mg, Fe, and Zn (Lizarazo et al., 2015; Longobardi et al., 2015; Neme et al., 2015). Faba bean seeds also contain several other bioactive compounds, such as polyphenols (Turco et al., 2016), carotenoids (Neme et al., 2015), and carbohydrates (Landry et al., 2016). However, the chemical composition is strongly influenced by variety, as well as environmental and management conditions (Mona et al., 2011; Cazzato et al., 2012; Witten et al., 2015).
The inclusion of faba bean in cropping systems improves soil fertility. Its high efficiency in establishing symbiosis with specific Rhizobium bacteria, and the concomitant biological nitrogen fixation (BNF), is associated with a reduced need for fertilizer input in arable lands and increased soil biological activity. The two main agricultural practices that benefit from BNF are crop rotation cycles that include legumes, and the intercropping of legumes with crops that are incapable of fixing N, such as cereals or horticultural crops (Jensen et al., 2010). The amount of nitrogen that can be fixed by faba bean is mainly dependent on the cultivar, local farming practices (such as nitrogen and phosphorus fertilization), soil properties, and the presence of symbiotically effective rhizobia in the soil (Schubert et al., 1990; Adak and Kibritci, 2016; Argaw and Mnalku, 2017).
Several studies have shown that incorporating legume residues into the soil as green manure increases growth and yield in many crops, such as canola, maize, potato, and wheat (Sincik et al., 2008; Miller et al., 2011; Bilalis et al., 2012; O’Donovan et al., 2014). According to Neugschwandtner et al. (2015), faba bean can fix up to 200 kg N ha-1, while the incorporation of legume residues into the soil improves soil properties such as organic matter content, bulk density, porosity, and field capacity (Mandal et al., 2003; Salahin et al., 2013; Adekiya et al., 2017). However, in spite of the multiple advantages of using faba bean in crop rotation systems, the area used for its cultivation has decreased in most European countries in comparison with the 1981–1990 period, as reported previously (FAO, 2017).
Given the importance of faba bean in agroecosystems, this review aims to provide an up-to-date overview of the genetic material, its nutritional value, cropping practices, and the role of this legume species in maintaining sustainability in agricultural production in Europe. Cultivation practices that could improve the production and nutritional value of faba bean are highlighted; the main constraints in its commercial production and an overview of its adaptability to different abiotic stresses are also presented. The importance of screening for and creating new varieties with an increased resilience to biotic and/or abiotic stress is emphasized.
History, Origin, And Distribution
The genus Vicia L. belongs to the family Fabaceae. Knowledge of the wild progenitor and area of origin of the genus, and subsequent steps in the domestication of its most important member species, V. faba L., is scarce and disputed (Shiran et al., 2014). The Near East is considered a center of origin for faba bean (Cubero, 1974), while China seems to be a secondary center of faba bean genetic diversity (Zong et al., 2009, 2010). In support of Cubero’s findings, Caracuta et al. (2016) have identified seeds of a potential ancestor of faba bean adjacent to Mount Carmel, Israel – the remains were C-dated to 14,000 years BP (before present). Moreover, Caracuta et al. (2015) have determined that faba bean was already domesticated about 10,200 years BP in the Lower Galilee, Israel. In any case, faba bean can be considered one of the earliest domesticated crops in light of numerous archeological findings in Eurasia and Africa which date back to the early Neolithic (Duc et al., 2015a).
Vicia faba has a large genetic diversity. According to Duc et al. (2010, 2015a), >38,000 accessions of faba bean germplasm are conserved globally in numerous gene banks, as well as at the International Center for Agricultural Research in Dry Areas (ICARDA). Research conducted by the EUROLEGUME consortium has shown that potentially many more genotypes are available locally in Europe, at farms and in breeders’ collections (Lepse et al., 2017); a small selection of faba bean genotypes of European origin are shown in Table 1. The genetic diversity of V. faba accessions has been assessed in various studies and marker systems (Zeid et al., 2003; Zong et al., 2009; Oliveira et al., 2016; Sallam et al., 2016; Göl et al., 2017). In practice, however, a continuous variation in most morphological, (eco-)physiological, and chemical traits has been observed, making it challenging to achieve a discrete differentiation between varieties. A study by Martos-Fuentes (2017) shows that NGS (next-generation sequencing)-based genotyping of faba bean using multiple barcoded samples is a feasible method. The author’s resulting distance matrix shows that some V. faba clades were exclusively formed by accessions from a specific country, while others were interspersed, indicating that genetic and geographic distances do not always correspond.
Table 1.
Selected Vicia faba var. minor and var. major genotypes originating from various European countries.
| Country of origin | Genotypes | Reference |
|---|---|---|
| Albania | BG 144001, BG 148005, BG 146003, BG 145002, BG 793710, BG 788700, BG 789701, BG 787699, BG 790705, BG 144001, BG 147004, AUT 0001, and AUT 0002 | Nasto et al., 2016 |
| Austria | Di-2384, Di-2385, and Di-2310 | Moussart et al., 2008 |
| Belgium | V-211 | Rubiales et al., 2014 |
| Bulgaria | V-294 | Rubiales et al., 2014 |
| Denmark | Di-2387 | Moussart et al., 2008 |
| France | Di-279, Di-1626, Di-276, Di-277, and Di-281 | Moussart et al., 2008 |
| Germany | Di-2436 | Moussart et al., 2008 |
| Greece | Ftakoukia, Platokoukia, Stenokoukia, AUAANDROSfb001, AUALEFKADAfb001, and AUAMANIfb001 | Thomas et al., 2013; Ntatsi et al., 2018 |
| Hungary | V-319 | Rubiales et al., 2014 |
| Netherlands | Di-1216 | Moussart et al., 2008 |
| Latvia | Bauska, Priekulu 32, Priekuïu vietejas, Valmiera, Džūkstes, Zaigas, Puntuïa tumŠās, Cēres, Puntuïa gaiŠās, Iras, VF_01, VF_02, and V-329 | Rubiales et al., 2014; Dane et al., 2017; Bodner et al., 2018 |
| Russia | V-1271 | Rubiales et al., 2014 |
| Spain | Di-1653, V-903, and V-1196 | Moussart et al., 2008; Rubiales et al., 2014 |
| Sweden | Gubbestad | Bodner et al., 2018 |
| Ukraine | V-268 | Rubiales et al., 2014 |
Evolution of the species was accompanied by intensified cultivation, with selection for different traits. The genotypes of V. faba are commonly classified into three main botanical varieties according to seed size: (a) V. faba var. major with large seeds, (b) V. faba var. minor with small seeds, and (c) V. faba var. equina with medium seeds (Cubero, 1974; Crépon et al., 2010; Pietrzak et al., 2016), the first two of which are relevant in European agriculture. However, faba bean germplasm is also grouped into spring and winter types, according to frost tolerance, delimiting target climatic zone, and sowing time, and according to the ability to adapt to oceanic or continental (i.e., drought-prone) climates (Moreno and Martinez, 1980; Link et al., 2010; Flores et al., 2013). Recently, Zhao et al. (2018) have shown that cultivar groups featuring differential root system architectures exist independently of botanical variety within Europe, but that, for example, cultivars from Portugal possess greater and coarser but less frequent lateral roots at the top of the taproot in comparison with Northern European cultivars, potentially enhancing water uptake from deeper soil horizons.
Morphological Description and Botanical Characterization
Faba bean is a cool season annual legume (Bilalis et al., 2003) that forms coarse, upright, hollow, and unbranched stem(s) from the base, and grows between 0.1 and 2 m tall (Bond et al., 1985; Duc et al., 2015a; Heuzé et al., 2016). Stem growth is indeterminate, and some cultivars are prone to lodging. The leaves are alternate, pinnate, and consist of two to six leaflets, which are up to 8 cm long without tendrils (Bond et al., 1985). The flowers have a typically papilionaceous structure and are grouped in inflorescences; they are either pure white in color or with diffuse anthocyanin pigmentation on all petals, while black spots are often present on the wing petals (Bond et al., 1985; Duc et al., 2015a; Heuzé et al., 2016). Seeds, which vary considerably in size, are oblong to broadly oval with a prominent hilum at the end; their color can be yellow, green, brown, black, or violet, and sometimes seeds are spotted (Nozzolillo et al., 1989; McDonald and Copeland, 1997; Duc et al., 2015a). Faba bean plants feature a robust taproot with frequent lateral root branching from the top of the tap root; nitrogen-fixing nodules containing rhizobia occur on both the tap and lateral roots (Bond et al., 1985). Root traits of European accessions vary profoundly (Zhao et al., 2018), and are largely influenced by the tillage regime (Muñoz-Romero et al., 2011).
Faba bean is generally considered day-neutral, while some accessions require long-day conditions in order to flower. However, thermal time is the most important contributor to flowering progress in faba bean, with approximately 830–1000°days above 0°C being required; winter faba bean genotypes require vernalization (Patrick and Stoddard, 2010). For northern European cropping systems, Bodner et al. (2018) have recently reported results of 650°days and 0°C base temperature before flowering; this potentially reflects a photoperiodic sensitivity toward long-day conditions in faba bean. In a recent study, Cao et al. (2017) found that several potential regulators are implicated in the vernalization process in faba bean. Faba bean is a self-pollinated plant with significant levels of cross-pollination (Suso et al., 1996; Chen, 2009). The main pollinating insects are honeybees (Apis spp.) and bumblebees (Bombus sp.); the benefits of insect pollination for yield have been well documented (Stoddard, 1991; Cunningham and Le Feuvre, 2013; Bishop et al., 2016).
Adaptability of Faba Bean to Abiotic Stress
Drought and heat are considered major constraints in faba bean growth and production in Europe. The most drought-sensitive growth stages are flowering, early podding, and grain filling (Figure 1; Mwanamwenge et al., 1999; Katerji et al., 2011). However, faba bean varieties differ widely in drought tolerance (Girma and Haile, 2014). One of the mechanisms apparent in drought-tolerant varieties or genotypes is proline accumulation (Migdadi et al., 2016; Abid et al., 2017), but a differential root architecture, influencing access to water, which differentiates varieties originating from Northern vs. Southern Europe, has also recently been identified (Zhao et al., 2018).
FIGURE 1.
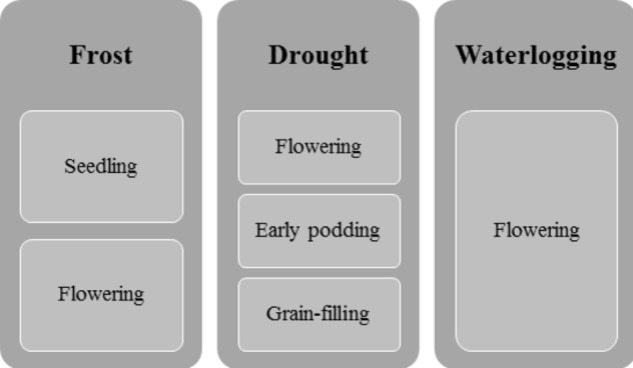
Critical stages of faba bean growth in responding to the main abiotic stress factors.
According to Maqbool et al. (2010), faba bean is susceptible to frost during its reproductive stages. Recent studies, however, have identified some frost-tolerant genotypes, which could be used in breeding programs (Stoddard et al., 2006; Sallam et al., 2015). Hardening seedlings through exposure to low non-freezing temperatures before the onset of winter may enhance plant tolerance to frost (Arbaoui and Link, 2008).
Waterlogging, e.g., during flowering, limits faba bean growth and yield (Pampana et al., 2016). The negative effects of waterlogging on growth and other physiological traits (i.e., chlorophyll a and b) persist even after cessation of soil flooding (Pociecha et al., 2008). However, faba bean is considered the most tolerant to waterlogging of the cool-season grain legumes (Solaiman et al., 2007).
Excess soil salinity affects both growth and nitrogen fixation in faba bean plants, which are considered moderately tolerant of soil salinity (Bulut et al., 2011). Katerji et al. (2011) report a yield reduction in faba bean at soil salinity levels of ≥6.5 dS m-1. According to del Pilar Cordovilla et al. (1999), root systems are more sensitive than shoots to salinity. In addition, at high salinity levels, nitrogenase activity and nodulation are suppressed (Abd-Alla et al., 2001). These researchers observe that high salinity treatments of 80 and 120 mM NaCl caused a significant reduction in faba bean shoot biomass of 25 and 49%, respectively. Moreover, nitrogen accumulation in the shoots reduced by 36 and 63% at salinity levels of 80 and 120 mM NaCl, respectively (Abd-Alla et al., 2001). Measures undertaken to ameliorate the negative effects of salinity on faba bean plants include foliar application of silicon or inoculation with Pseudomonas fluorescens (Hellal et al., 2012; Metwali et al., 2015). Salinity-tolerant faba bean genotypes are also available; one example is the line “VF112,” which has been reported as salt-tolerant because salt stress had no effect on its growth or nitrogen fixation (del Pilar Cordovilla et al., 1995). Examples of genotypes with enhanced tolerance to abiotic stresses are presented in Table 2.
Table 2.
Responses of different Vicia faba genotypes to abiotic and biotic stresses.
| Genotypes | Type of abiotic stress | Level | Reference |
|---|---|---|---|
| Boxer | Heat stress | T | Zhou et al., 2018 |
| FAB6600 | Cold stress | S | Zhou et al., 2018 |
| NGB8639 | Cold stress | T | Zhou et al., 2018 |
| FAB7024 | Cold stress | T | Zhou et al., 2018 |
| S_145, S_004, S_081, S_151, S_299 | Frost stress | T | Sallam et al., 2017 |
| S_165, S_129, S_232, S_235, S_111 | Frost stress | S | Sallam et al., 2017 |
| C5 | Drought stress | T | Siddiqui et al., 2015 |
| Zafar 1 | Drought stress | T | Siddiqui et al., 2015 |
| C4, G853 | Drought stress | S | Siddiqui et al., 2015 |
| CS20-DK and NC-58 | Drought stress | T | Girma and Haile, 2014 |
| Giza 3 | Drought stress | S | Abid et al., 2017 |
| Hara | Drought stress | T | Abid et al., 2017 |
| Fiesta VF, Acc 1487/7, Acc 1512/2 | Salt stress | T | Tavakkoli et al., 2012 |
| VF46, VF64, and VF112 | Salt stress | T | del Pilar Cordovilla et al., 1995 |
| Baraca | Orobanche crenata | T | Rubiales et al., 2014 |
| V-26, V-255, V-958, V-1020, V-1085, V-1117, and L-831818 | Ascochyta fabae | T | Rubiales et al., 2012 |
| BPL 710, ILB 4726, ILB 5284, 132-1, 135-1, 174-1 | Botrytis fabae | T | Villegas-Fernández et al., 2012 |
T, Tolerant; S, sensitive.
Nitrogen fixation and growth in faba bean are adversely affected by low soil pH (Schubert et al., 1990; Belachew and Stoddard, 2017). Faba bean grows best in soils with a pH ranging from 6.5 to 9.0 (Jensen et al., 2010). At low pH levels (<5.4), BNF is significantly lower than at higher pH levels (>6.2); at pH levels of <4.7, plants show N deficiency symptoms during the early growth stages (Schubert et al., 1990).
Agronomy
Crop Sowing and Rotation
Faba bean is usually planted in the autumn, in areas of Europe characterized by mild winter climatic conditions (Bilalis et al., 2003). In cooler agroclimatic zones, sowing is postponed until the end of winter or early spring to prevent frost damage (Sallam et al., 2015). In some areas of the Mediterranean Basin, the earliest varieties can be sown at the end of summer, with the aim of harvesting them by the end of autumn (Cubero, 2017).
The main tillage operations during the sowing period include moldboard plowing (20–40 cm depth) and harrowing, followed by light duty plowing, the last of which is commonly performed using a rotary tiller. Several studies also show that reduced tillage and no-tillage are viable alternatives to conventional tillage in faba bean crops (López-Bellido et al., 2003; Lestingi et al., 2011; Muñoz-Romero et al., 2011; Giambalvo et al., 2012).
Faba bean is usually sown in rows 10–30 cm apart (Bozoğlu et al., 2002; Yucel, 2013), using either a spacing drill (placing 2–3 seeds per hole) or seed drill. The required seed amount ranges between 70 and 200 kg ha-1, dependent on seed size and planting density. According to Siddique and Loss (1999), the recommended sowing depth is 5–8 cm. Germination takes place in 4–12 days, and the optimum temperature for germination is 20°C (Khamassi et al., 2013a).
The key agronomic and economic advantage provided by faba bean and other legumes in crop rotation is BNF (Köpke and Nemecek, 2010). The N benefit provided for subsequent crops is often high; a review by Jensen et al. (2010) has demonstrated substantial savings (up to 100–200 kg N ha-1) in the amount of N fertilizer required for subsequent crops. Thus, the inclusion of faba bean in crop rotation reduces the need for inorganic N fertilizer, and consequently reduces CO2 emissions (Jensen et al., 2012). Other benefits provided by faba bean in rotation systems include improvement to soil physical properties, maintenance of soil fertility, and disruption of pest and disease cycles (Chalk, 1998; Mandal et al., 2003; Stoddard et al., 2010; Adekiya et al., 2017). Nevertheless, there are some environmental risks, such as increases in N leaching or N2O emissions, associated with the use of faba bean in crop rotation; these risks can however be limited through appropriate rotation system design (Huth et al., 2010).
In summary, the main benefits of including faba bean in crop rotation systems are as follows: (1) reduced use of inorganic nitrogen fertilizers, (2) reduced CO2 emissions, (3) improved soil physical properties (i.e., bulk density, porosity, and water content at field capacity), (4) maintenance of soil fertility, and (5) higher yield and improved quality in subsequent crops.
Faba bean is usually employed as a break crop in cereal production. When rotated with cereals, it has proven to be beneficial in increasing the yield and seed protein content of successive cereal crops (Zou et al., 2015). In some Mediterranean countries, it is also incorporated into vegetable crop rotations, e.g., it can be utilized as a pre-crop of some summer crops, such as species in the Cucurbitaceae or Solanaceae families. Below are two examples of rotation sequences used in European cropping systems that include faba bean:
-
simple 1.
Faba bean (first year), cereal (second year), field or industrial crops (i.e., maize, cotton, tomato, sugar beet, oilseed rape; third year), and cereal (fourth year);
-
simple 2.
Faba bean – short period vegetable (first year), cereal (second year), field or industrial crops (i.e., maize, cotton, tomato, sugar beet, oilseed rape; third year), and cereal (fourth year).
Soil Fertilization and Inoculation
Nitrogen fertilization is not generally required, but the application of “starter” nitrogen fertilization at a rate of 20 kg ha-1 seems to enhance the nodulation process in faba bean plants (Mohamed and Babiker, 2012). Furthermore, legume BNF is an energy intensive process that requires large amounts of phosphorus (P). Thus, P fertilization at a rate of 40 kg ha-1 can often enhance the nodulation process and N2 fixation, and increase yield (Bolland et al., 2000; Adak and Kibritci, 2016). Several other studies show that faba bean crops also respond to S and K fertilization (Sangakkara et al., 1996; Niewiadomska et al., 2015). Nevertheless, S or K fertilizers are rarely applied, because faba bean is cultivated as a low-input crop. Furthermore, micronutrient (e.g., zinc and boron) deficiencies are rare and can easily be corrected through foliar sprays.
Inoculating faba bean fields or seeds with Rhizobium is unnecessary in traditional cultivation areas. However, it is advisable to test their presence in the soil in areas where faba beans or other legumes have not been grown for several years. If absent, the crop can be inoculated with Rhizobium leguminosarum bv. viciae (Cubero, 2017). Dual inoculation with Rhizobium and arbuscular mycorrhizal fungi has been reported to be more effective than inoculation with Rhizobium alone in promoting faba bean growth, particularly in alkaline soils; this reflects the existence of synergistic relationships between the two inoculants (Abd-Alla et al., 2014).
Irrigation
Faba bean usually grows without irrigation, with the exception of crops cultivated in very dry and hot climatic zones. Thus, production is highly dependent on the amount of and variation in rainfall during the growing season (Oweis et al., 2005). In semiarid regions, climate change can affect water use efficiency and growth in faba bean (Guoju et al., 2016), given its sensitivity to drought (Ghassemi-Golezani et al., 2009; Alghamdi et al., 2015). In the Mediterranean region and similar dry and hot climatic zones, faba bean production without irrigation may be possible if cultivation takes place during the cold season. Moreover, early sowing in autumn is considered an effective strategy for avoiding water stress during the seed filling stage (Loss and Siddique, 1997). Alternatively, faba bean crops can be irrigated at the seed filling stage in order to avoid penalties in yield during drought. Additionally, Knott (1999) reports that faba bean production is usually increased by irrigating spring crops during the flowering stage and early podding. Between 231 and 297 mm of water are required to produce 3–4.4 t ha-1 of faba bean dry biomass (Bryla et al., 2003). The development of drought-tolerant faba bean varieties is a key challenge in achieving increased and more stable production levels (Khan et al., 2010; Siddique et al., 2013). Several genotypes are considered tolerant to drought and can be exploited in breeding programs in order to develop drought-tolerant varieties (Ali, 2015). Recently, some varieties (e.g., CS20-DK and NC-58) have been evaluated as tolerant to water stress (Girma and Haile, 2014).
Weed Control
Weed infestation is a major constraint in faba bean production, and can reduce yield by up to 50% (Frenda et al., 2013). Thus, early weed removal during the period between 25 and 75 days after sowing is necessary if a high yield is to be obtained (Tawaha and Turk, 2001). Similar to other winter pulse crops and cereals, the 12 main weeds that compete with faba bean in Europe are the broadleaved species Anthemis arvensis L., Chenopodium album L., Papaver rhoeas L., Sinapis arvensis L., Fumaria officinalis L., Veronica spp., Lamium amplexicaule L., Cirsium arvense (L.) Scop., and the grass species Avena sterilis L., Phalaris spp., Lolium rigidum Gaud., and Alopecurus myosuroides Huds. (Kalburtji and Mamolos, 2001; Karkanis et al., 2016a,b). Moreover, in many Mediterranean countries, such as Spain, faba bean can be parasitized by various broomrape species (Orobanche spp. and Phelipanche spp.) (Pérez-de-Luque et al., 2010); Orobanche crenata Frosk (bean broomrape) is the main species infesting faba bean in this area (Pérez-de-Luque et al., 2016).
Faba bean exhibits a superior ability to compete with weeds compared with other pulse crops, such as chickpea, due to its more vigorous early growth and greater plant height (Frenda et al., 2013). Nevertheless, the application of herbicides is a primary method in controlling weeds in conventional faba bean production. To our knowledge, the herbicides pendimethalin, clomazone, bentazon, quizalofop-p-ethyl, and propaquizafop are registered for use on this crop in the European Union. The first two are applied pre-emergence to control broadleaved and grass weeds; quizalofop-p-ethyl and propaquizafop are applied post-emergence to control grass weeds such as Phalaris spp. and Lolium spp., while bentazon is applied post-emergence to control broadleaved weeds. Crop rotation with spring crops can significantly reduce weed pressure, while allowing field application of herbicides that are not registered for use on faba bean (Karkanis et al., 2016b). Residual herbicides can damage faba bean planted in fields where chlorsulfuron (sulfonylureas) and aminopyralid (pyridine carboxylic acids) have previously been applied.
Currently, the development of resistant faba bean varieties would appear to be the most effective strategy for preventing broomrape infestation. In a recent study conducted in Egypt, Spain, and Tunisia by Rubiales et al. (2014), some accessions and the variety “Baraca” proved to be the most resistant to O. crenata. Several studies have also shown that late sowing and intercropping with cereals can reduce broomrape infection of faba bean (Pérez-de-Luque et al., 2004; Fernández-Aparicio et al., 2007; Abbes et al., 2010), while soil solarization is a non-chemical and effective method for controlling O. crenata and other weeds (Haidar and Sidahmed, 2000; Mauromicale et al., 2001).
Disease and Insect Management
Diseases
Fungal diseases can severely damage faba bean crops, especially in wet weather conditions. Ascochyta blight, chocolate spot, and rust are the three main pathogens affecting faba bean crops globally (Torres et al., 2006; Stoddard et al., 2010). Ascochyta blight is caused by Ascochyta fabae Speg. (teleomorph Didymella fabae Jellis and Punithalingam), and is one of the most serious pathogens, causing up to 30% loss in yield (Davidson and Kimber, 2007; Omri Benyoussef et al., 2012; Ahmed et al., 2016). Although the application of fungicides, such as azocystrobin and chlorothalonil, considerably reduces ascochyta blight infection, integrated management practices (e.g., crop rotation, use of resistant varieties, and late sowing) are crucial to successful control (Davidson and Kimber, 2007; Stoddard et al., 2010; Ahmed et al., 2016). In a recent study, Rubiales et al. (2012) report that the faba bean accessions V-26, V-255, V-958, V-1020, V-1085, V-1117, and L-831818 showed good levels of resistance to A. fabae.
Chocolate spot is caused by the fungi Botrytis fabae Sard. and Botrytis cinerea Pers., while Uromyces viciae-fabae (Pers.) J. Schröt causes rust disease in faba bean (Emeran et al., 2011; Fernández-Aparicio et al., 2011; Abd El-Rahman and Mohamed, 2014). Rust and chocolate spot infection can cause yield losses of 22–42% and 36–68%, respectively (Sahile et al., 2010; Emeran et al., 2011). According to Emeran et al. (2011), foliar spraying with fungicides such as the triazoles (difenoconazole, epoxiconazole, or tebuconazole), dithiocarbamates (thiram, maneb, or mancozeb), and chlorothalonil was effective in controlling rust. In addition, procymidone is very effective against B. fabae (Marcellos et al., 1995), and chocolate spot severity in faba bean is reduced by frequent application of mancozeb (Sahile et al., 2008), intercropping with cereals such as barley, oat, triticale, and wheat (Sahile et al., 2008; Fernández-Aparicio et al., 2011), and low crop density and wide row spacing (Davidson et al., 2007). In a recent study, Mbazia et al. (2016) also observe that isolates of Trichoderma viride, T. harzianum, and Bacillus subtilis reduced chocolate spot severity in faba bean. A key option in the integrated management of B. fabae is the cultivation of resistant cultivars. According to Villegas-Fernández et al., 2012), the accessions 132-1, 135-1, 174-1, BPL 710, ILB 4726, and ILB 5284 exhibited a good level of resistance to B. fabae infection. Thus, these genotypes constitute an interesting genetic resource for future exploitation in breeding programs for developing chocolate spot-resistant cultivars.
Faba bean is also susceptible to viruses, with the principal sources of infection being faba bean necrotic yellows virus (FBNYV) and bean yellow mosaic virus (BYMV) (Ortiz et al., 2006; Shiying et al., 2007). Other diseases affecting faba bean crops are black root rot [Fusarium solani (Mart.) Sacc.], faba bean root rot (Aphanomyces euteiches Drechs.), powdery mildew (Erysiphe pisi var. pisi), and stem rot (Sclerotinia trifoliorum Erikss.) (Cook and Fox, 1992; Lithourgidis et al., 2003; van Leur et al., 2008; Habtegebriel and Boydom, 2016).
Insects
Several insects have the potential to infest faba bean plants. The black bean aphid (Aphis fabae Scop.) is a common pest (Hansen et al., 2008); aphids infest new leaves on faba bean plants (Nuessly et al., 2004). Foliar insecticide sprays (i.e., thiacloprid, fenvalerate) are very effective against these pests (Purhematy et al., 2013). Moreover, parasitoids play a significant role in the natural control of aphids (Boivin et al., 2012). Lysiphlebus fabarum Marshall (Hymenoptera) is a parasitoid of black bean aphid, and could prove useful as a biological control (Mahmoudi et al., 2010).
Other insects that infect faba bean crops are the pea leaf weevil (Sitona lineatus L.) and broad bean weevil (Bruchus rufimanus Boh.; Evenden et al., 2016; Seidenglanz and Huņady, 2016). S. lineatus adults feed on the foliage, while the larvae feed on faba bean and pea root nodules, affecting their ability to fix nitrogen (Cárcamo et al., 2015); treating seeds with thiamethoxam could be useful in controlling this insect (Cárcamo et al., 2012). Furthermore, storage pests, such as B. rufimanus, can cause significant yield losses in legumes; insecticides are however effective against them (Keneni et al., 2011).
Genetic Material: Yield
Faba bean has a long history of cultivation. A broad gene pool has therefore been developed over several centuries, including local landraces, mass selections from landraces, open-pollinated populations, inbred lines, and cultivars (Duc et al., 2010). In addition, socioeconomic changes have led to decreases in cultivation and the disappearance of local genetic resources, with only small farms continuing to grow different landraces selected for their adaptation to local environmental conditions (Karaköy et al., 2014).
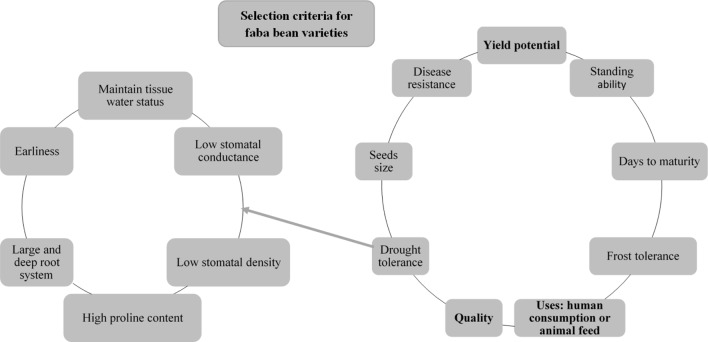
Investment in legume breeding has been lower than for cereals (Fouad et al., 2013; Duc et al., 2015b) and, as a result, only a limited number of registered faba bean cultivars is available. For example, only 256 faba bean cultivars are currently registered for growing in Europe, and recorded at the EU database of registered plant varieties; for wheat (Triticum aestivum); however, there are 2415 registered cultivars (European Commission, 2017). “Aguadulce,” “Extra,” “Precose,” “Tundra,” “Fuego,” “Extra Violetto,” “Babylon,” and “Pyramid” are some commercial varieties cultivated in Europe under a wide range of agroclimatic conditions. Registered varieties feature a range of highly differing characteristics although genetic variation is limited (Fouad et al., 2013). Traits that should be targeted in selecting faba bean varieties include yield potential, quality, consistent performance, suitability for human consumption or the feed market, seed size, days to maturity, and standing ability (Figure 2), as well as resistance to disease and abiotic stress (see above sections).
FIGURE 2.
Main selection criteria for faba bean varieties (right-hand side); specific criteria for drought tolerance are indicated in detail (left-hand side).
The primary focus of legume breeding, including faba bean, is yield. However, in many regions, faba bean crops are subject to different conditions of biotic and abiotic stress and, consequently, yield is ultimately dependent on cultivar resilience to multiple stress conditions. Hence, breeding new cultivars with increased resilience to abiotic stresses, such as heat and salinity, continues to challenge breeders (Siddique et al., 2013; Nebiyu et al., 2016). Furthermore, winter hardiness is an important trait in screening for cultivars to be cultivated during the cold season (Link et al., 2010).
The genetic improvement of desired traits via breeding significantly depends on genetic variation in those traits. There is therefore an urgent need to collect and evaluate local genetic resources that can be used in well-designed breeding programs as donors of valuable features in the development of new improved varieties. In this sense, local landraces represent important sources for plant breeding, as they contain co-adapted genes that may prove valuable in future cultivation practices and in enhancing yield and quality (Harlan, 1975). The importance and wide variation of traits relating to morphology, agronomy, and quality have been previously investigated and demonstrated for local faba bean genetic resources of different origins: Turkey (Karaköy et al., 2014), the Mediterranean (Terzopoulos and Bebeli, 2008), Albania (Nasto et al., 2016), Palestine (Basheer-Salimia et al., 2014), and China (Zong et al., 2009). However, only a small proportion of other faba bean genetic resources has so far been evaluated. Thus, efforts to characterize the available resources should be intensified, and the collection of new local resources is crucial, because of the genetic erosion that is currently identified. Furthermore, breeding programs need to incorporate a more complex evaluation and integrated use of traits (Duc et al., 2015b). The abovementioned traits and others, such as root architecture (Zhao et al., 2018), shoot architecture, parameters related to stomatal function (Khan et al., 2010; Khazaei et al., 2013), and multiple disease resistance (Torres et al., 2006), are becoming increasingly important. It is apparent that any new variety should combine as many of the abovementioned characteristics as possible, in order to allow for a greater and more stable production of faba bean in specific agroecological zones.
The anticipated dry seed yield in faba bean crops ranges between 1.6 and 5.2 tons ha-1 (Argaw and Mnalku, 2017; Youseif et al., 2017), and the fresh pod yield ranges between 1.34 and 17.04 tons ha-1 (Baginsky et al., 2013; Etemadi et al., 2017). Faba bean yield components, such as the thousand seed weight, number of pods per plant, and number of seeds per pod, together with the duration of phenological stages and plant height, are correlated to grain yield (Sharifi, 2014; Bodner et al., 2018).
Yield stability and quality is a major objective of faba bean breeders (Alghamdi et al., 2012; Flores et al., 2013), since yield instability is a common problem encountered in cultivating this species and is considered a main cause of the decline in faba bean acreage (Suso et al., 1996; Flores et al., 2013). The stability of faba bean genotypes in different environmental conditions also needs to be examined (Temesgen et al., 2015). Several approaches can be applied in evaluating yield stability. Temesgen et al. (2015) demonstrate that different stability parameters have varying effects on yield performance, and recommend the application of several stability parameters, rather than only considering yield in different years. Faba bean genotypes exhibit a strong interaction with environmental conditions (Flores et al., 2013; Maalouf et al., 2015; Temesgen et al., 2015). In their study, Temesgen et al. (2015) report that the environment (E) accounted for 89% of yield variation, while the genotype (G) and the “G × E” interaction contributed 2 and 3%, respectively. The breeding of genotypes adapted to specific climatic zones is recommended in order to increase yield stability (Flores et al., 2013). G × E interactions are more common in faba bean than in most other crops; Bond (1987) reports that genotype × season interactions generally make a greater contribution than genotype × location.
Harvest, Processing, Nutritional Value, and Use of Faba Bean
Faba bean crops cultivated for fresh seed consumption may be harvested either manually or mechanically once the pods are filled, but before they start to dry. Pods are harvested by hand two to three times during the harvesting period in crops cultivated in small areas for fresh consumption. When faba bean plants are cultivated for their dry seeds, they can be harvested using a conventional cereal combine harvester. Similar to other pulses, proper selection of the harvest stage is critical if seed loss is to be minimized (Karkanis et al., 2016a); seeds should be harvested when the moisture content is 14–15% (Jilani et al., 2012). In some countries (such as Canada), diquat is registered as a preharvest desiccant, and its application is a common practice among pulse growers (McNaughton et al., 2015), as this helps farmers to overcome problems caused by slow ripening and weeds during the harvest period.
Faba bean seeds also contain antinutrient compounds. Soaking, dehulling, boiling, pressure-cooking, autoclaving, and extrusion cooking are the main processing methods used to reduce the amounts of these compounds in faba bean seeds, in order to limit their adverse effects on human health (Luo and Xie, 2013; Patterson et al., 2017; Shi et al., 2017). Dehulling is efficient in eliminating the tannin and polyphenol content (Alonso et al., 2000), while soaking and autoclaving inactivate trypsin inhibitor activity (Luo and Xie, 2013; Shi et al., 2017).
The inclusion of plant-based proteins in human diets has a beneficial effect on human health (Moorthi et al., 2015). Faba bean protein content is reported to vary between 17.6 and 34.5% of seed dry matter, while acid detergent fiber (ADF) ranges between 10.1 and 13.7% (Table 3). Faba bean is also a valuable source of amino acids, being particularly rich in the essential amino acids arginine, lysine, and leucine, at up to 67 g kg-1 dry matter (Koivunen et al., 2016). As faba bean also provide macro-, micro-, and non-nutrient phytochemicals, it has been noted to have potential as a functional food. For example, Brauckmann and Latté (2010) report that faba bean seeds contain L-3,4-dihydroxyphenylalanine (L-DOPA), the precursor to the neurotransmitter catecholamine and a drug used to treat Parkinson’s disease.
Table 3.
Nutritional value of faba bean dry seeds in comparison with two other important legumes widely cultivated in Europe.
ADF, acid detergent fiber; NDF, neutral detergent fiber.
Faba bean also contains antinutritional compounds such as saponins, lectins, tannins, vicine, convicine, and phytic acid (Hendawey and Younes, 2013; Multari et al., 2015). Tannins are known to reduce protein digestibility, while the absence of tannin in zero-tannin faba beans is controlled by either of the two genes zt-1 and zt-2 (Gutierrez et al., 2008; Woyengo and Nyachoti, 2012). The consumption of faba bean products containing high levels of vicine and convicine causes favism in humans, which is associated with glucose-6-phosphate dehydrogenase deficiency (Khamassi et al., 2013b).
Faba bean seed size is an important trait in determining market and consumption form. Large-seeded varieties (broad beans) are widely used for food, either as a fresh green vegetable or (dehulled) dry seeds. Varieties with small- to medium-size seeds are mostly used for animal feed (Crépon et al., 2010). Faba bean can also be used in the bakery industry (Belghith-Fendri et al., 2016); for example, a combination of faba bean and wheat flour improves the nutritional properties of bread (Coda et al., 2017). In Spain, small faba bean seeds (<12 mm) are currently highly accepted in the industry (Cubero, 2017). Small-seed genotypes are generally preferred by the frozen faba bean (Baginsky et al., 2013) and canning industries; the ability to use a microwave oven encourages the consumption of this legume, because seeds are much more easily cooked, and bags can be stored for up to 10 days at 5°C (Collado et al., 2017).
Conclusion
Faba bean is important both as a pulse and a vegetable crop. The dry and fresh seeds or pods are recommended for their benefits to human nutrition as a dietary source of fiber and protein. Moreover, from an agronomical point of view, including faba bean in crop rotation systems improves soil, since this crop can fix atmospheric N2 to amounts that may exceed 200 kg N ha-1, and increases soil organic matter. Its inclusion in rotation systems therefore contributes to significant improvements in the sustainability of agricultural systems.
Fewer varieties of faba bean are recorded in comparison with other species in the European Union database. Production of this legume species is vulnerable to biotic and abiotic stresses, such as ascochyta blight, broomrape infestation, waterlogging, and drought. These constraints require there to be an urgent and increased focus on the development of new varieties that are resilient to these stresses. The new varieties should combine many of the above-mentioned characteristics, with the ultimate objective of achieving a high yield and high protein content.
Author Contributions
AK, GN, ER, and DS developed the initial concept and outline, expanding the content, edited and revised the paper. DS, ER, and AB wrote the sections “Introduction,” “Irrigation,” and “Conclusions.” BR and GB wrote the sections: “Morphological description and botanical characterization” and “History, origin, and distribution.” LL wrote the section “Nutritional value and use of faba bean.” GN wrote the section “Faba bean adaptability to abiotic stress.” JF and MO wrote the section “Crop sowing and rotation.” IA wrote the section “Soil fertilization and inoculation.” AK wrote the sections “Weed control,” “Disease,” and “Insect management.” AKr, MO, LD, GN, and IV wrote the sections “Genetic material: yield, harvest, and processing of faba bean.”
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by the European Commission as part of the project “EUROLEGUME-Enhancing of legumes growing in Europe through sustainable cropping for protein supply for food and feed (FP7 Research Project No. 613781).”
References
- Abbes Z., Sellami F., Amri M., Kharrat M. (2010). Effect of sowing date on Orobanche foetida infection and seed yield of resistant and susceptible faba bean cultivars. Acta Phytopathol. Entomol. Hung. 45 267–275. 10.1556/APhyt.45.2010.2.3 [DOI] [Google Scholar]
- Abd El-Rahman S. S., Mohamed H. I. (2014). Application of benzothiadiazole and Trichoderma harzianum to control faba bean chocolate spot disease and their effect on some physiological and biochemical traits. Acta Physiol. Plant. 36 343–354. 10.1007/s11738-013-1416-5 [DOI] [Google Scholar]
- Abd-Alla M. H., El-Enany A. E., Hamada A. M., Abdel Wahab A. M. (2001). Element distribution in faba bean root nodules under salinity and its effects on growth, nodulation and nitrogen fixation. Plant Soil Environ. 47 399–404. [Google Scholar]
- Abd-Alla M. H., El-Enany A. E., Nafady N. A., Khalaf D. M., Morsy F. M. (2014). Synergistic interaction of Rhizobium leguminosarum bv. viciae and arbuscular mycorrhizal fungi as a plant growth promoting biofertilizers for faba bean (Vicia faba L.) in alkaline soil. Microbiol. Res. 169 49–58. 10.1016/j.micres.2013.07.007 [DOI] [PubMed] [Google Scholar]
- Abid G., M’hamdi M., Mingeot D., Aouida M., Aroua I., Muhovsk Y., et al. (2017). Effect of drought stress on chlorophyll fluorescence, antioxidant enzyme activities and gene expression patterns in faba bean (Vicia faba L.). Arch Agron. Soil Sci. 63 536–552. 10.1080/03650340.2016.1224857 [DOI] [Google Scholar]
- Adak M. S., Kibritci M. (2016). Effect of nitrogen and phosphorus levels on nodulation and yield components in faba bean (Vicia faba L.). Legume Res. 39 991–994. [Google Scholar]
- Adekiya A. O., Agbede T. M., Aboyeji C. M., Dunsin O., Ugbe J. O. (2017). Green manures and NPK fertilizer effects on soil properties, growth, yield, mineral and vitamin C composition of okra (Abelmoschus esculentus (L.) Moench). J. Saudi Soc. Agric. Sci. (in press). 10.1016/j.jssas.2017.05.005 [DOI] [Google Scholar]
- Ahmed S., Abang M. M., Maalouf F. (2016). Integrated management of Ascochyta blight (Didymella fabae) on faba bean under Mediterranean conditions. Crop Prot. 81 65–69. 10.1016/j.cropro.2015.12.013 [DOI] [Google Scholar]
- Alghamdi S. S., Al-Shameri A. M., Migdadi H. M., Ammar M. H., El-Harty E. H., Khan M. A., et al. (2015). Physiological and molecular characterization of Faba bean (Vicia faba L.) genotypes for adaptation to drought stress. J. Agron. Crop Sci. 201 401–409. 10.1111/jac.12110 [DOI] [Google Scholar]
- Alghamdi S. S., Migdadi H. M., Ammar M. H., Paull J. G., Siddique K. H. M. (2012). Faba bean genomics: current status and future prospects. Euphytica 186 609–624. 10.1007/s10681-012-0658-4 [DOI] [Google Scholar]
- Ali M. B. (2015). Physiological response of German winter faba bean (Vicia faba L.) to drought. J. Crop Improv. 29 319–332. 10.1080/15427528.2015.1022918 [DOI] [Google Scholar]
- Alonso R., Aguirre A., Marzo F. (2000). Effects of extrusion and traditional processing methods on antinutrients and in vitro digestibility of protein and starch in faba and kidney beans. Food Chem. 68 159–165. 10.1016/S0308-8146(99)00169-7 [DOI] [Google Scholar]
- Arbaoui M., Link W. (2008). Effect of hardening on frost tolerance and fatty acid composition of leaves and stems of a set of faba bean (Vicia faba L.) genotypes. Euphytica 162 211–219. 10.1007/s10681-007-9521-4 [DOI] [Google Scholar]
- Argaw A., Mnalku A. (2017). Effectiveness of native Rhizobium on nodulation and yield of faba bean (Vicia faba L.) in Eastern Ethiopia. Arch. Agron. Soil Sci. 63 1390–1403. 10.1080/03650340.2017.1287353 [DOI] [Google Scholar]
- Baginsky C., Silva P., Auza J., Acevedo E. (2013). Evaluation for fresh consumption of new broad bean genotypes with a determinate growth habit in central Chile. Chil. J. Agr. Res. 73 225–232. 10.4067/S0718-58392013000300004 [DOI] [Google Scholar]
- Baloch F. S., Karaköy T., Demirbaş A., Toklu F., Özkan H., Hatipoglu R. (2014). Variation of some seed mineral contents in open pollinated faba bean (Vicia faba L.) landraces from Turkey. Turk. J. Agric. For. 38 591–602. 10.3906/tar-1311-31 [DOI] [Google Scholar]
- Basheer-Salimia R., Camilli B., Scacchi S., Noli E., Awad M. (2014). Genetic diversity among Palestinian faba bean (Vicia faba L.) ecotypes based on single nucleotide polymorphisms. Euro. J. Hort. Sci. 79 300–305. [Google Scholar]
- Belachew K. Y., Stoddard F. L. (2017). Screening of faba bean (Vicia faba L.) accessions to acidity and aluminium stresses. PeerJ 5:e2963 10.7717/peerj.2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belghith-Fendri L., Chaari F., Kallel F., Zouari-Ellouzi S., Ghorbel R., Besbes S., et al. (2016). Pea and broad bean pods as a natural source of dietary fiber: the impact on texture and sensory properties of cake. J. Food Sci. 81 C2360–C2366. 10.1111/1750-3841.13448 [DOI] [PubMed] [Google Scholar]
- Bilalis D., Karkanis A., Sidiras N., Travlos I., Efthimiadou A., Thomopoulos P., et al. (2012). Maize and legumes root growth and yield as influenced by organic fertilization, under mediterranean environmental conditions. Rom. Agric. Res. 211–217. [Google Scholar]
- Bilalis D., Sidiras N., Economou G., Vakali C. (2003). Effect of different levels of wheat straw soil surface coverage on weed flora in Vicia faba crops. J. Agron. Crop Sci. 189 233–241. 10.1046/j.1439-037X.2003.00029.x [DOI] [Google Scholar]
- Bishop J., Jones H. E., Lukac M., Potts S. G. (2016). Insect pollination reduces yield loss following heat stress in faba bean (Vicia faba L.). Agric. Ecosyst. Environ. 220 89–96. 10.1016/j.agee.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodner G., Kronberga A., Lepse L., Olle M., Vagen I. M., Rabante L., et al. (2018). Trait identification of faba bean ideotypes for Northern European environments. Eur. J. Agron. 96 1–12. 10.1016/j.eja.2018.02.008 [DOI] [Google Scholar]
- Boivin G., Hance T., Brodeur J. (2012). Aphid parasitoids in biological control. Can. J. Plant Sci. 92 1–12. 10.4141/cjps2011-045 [DOI] [Google Scholar]
- Bolland M. D. A., Siddique K. H. M., Brennan R. F. (2000). Grain yield responses of faba bean (Vicia faba L.) to applications of fertiliser phosphorus and zinc. Austral. J. Exp. Agric. 40 849–85. 10.1071/EA99164 [DOI] [Google Scholar]
- Bond D. A. (1987). Recent developments in breeding field beans (Vicia faba L.). Plant Breeding. 99 1–26. 10.1111/j.1439-0523.1987.tb01144.x [DOI] [Google Scholar]
- Bond D. A., Lawes D. A., Hawtin G. C., Saxena M. C., Stephens J. S. (1985). “Faba bean (Vicia faba L.),” in Grain Legume Crops, eds Summerfield R.J., Roberts E.H. (London: William Collins Sons; ), 199–265. [Google Scholar]
- Bozoğlu H., Pekşen A., Pekşen E., Gülümser A. (2002). Determination of green pod yield and some pod characteristics of faba bean (Vicia faba L.) cultivar/lines grown in different row spacings. Acta Hort. 579 347–350. 10.17660/ActaHortic.2002.579.58 [DOI] [Google Scholar]
- Brauckmann B. M. and Latt, é K. P. (2010). L-Dopa deriving from the beans of Vicia faba and Mucuna pruriens as a remedy for the treatment of Parkinson’s disease. Schweiz Z Ganzheitsmed. 22 292–300. [Google Scholar]
- Bryla D. R., Bañuelos G. S., Mitchell J. P. (2003). Water requirements of subsurface drip-irrigated faba bean in California. Irrig. Sci. 22 31–37. 10.1007/s00271-003-0065-7 [DOI] [Google Scholar]
- Bulut F., Akinci S., Eroglu A. (2011). Growth and uptake of sodium and potassium in broad bean (Vicia faba L.) under salinity stress. Commun. Soil Sci. Plant Anal. 42 945–961. 10.1080/00103624.2011.558963 [DOI] [Google Scholar]
- Çalıcskantürk Karataş S. C., Günay D., Sayar S. (2017). In vitro evaluation of whole faba bean and its seed coat as a potential source of functional food components. Food Chem. 230 182–188. 10.1016/j.foodchem.2017.03.037 [DOI] [PubMed] [Google Scholar]
- Cao Y. -Y., Bian X. -C., Chen M. -X., Xia L. R., Zhang J., Zhu F. -Y., et al. (2017). iTRAQ-based quantitative proteomic analysis in vernalization-treated faba bean (Vicia faba L.). PLoS One 12:e0187436. 10.1371/journal.pone.0187436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracuta V., Barzilai O., Khalaily H., Milevski I., Paz Y., Vardi J., et al. (2015). The onset of faba bean farming in the Southern Levant. Sci. Rep. 5:14370. 10.1038/srep14370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracuta V., Weinstein-Evron M., Kaufman D., Yeshurun R., Silvent J., Boaretto E. (2016). 14,000-year-old seeds indicate the Levantine origin of the lost progenitor of faba bean. Sci. Rep. 6:37399. 10.1038/srep37399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárcamo H. A., Herle C. E., Lupwayi N. Z. (2015). Sitona lineatus (Coleoptera: Curculionidae) larval feeding on Pisum sativum L. affects soil and plant nitrogen. J. Insect Sci. 15:74. 10.1093/jisesa/iev055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárcamo H., Herle C., Hervet V. (2012). Greenhouse studies of thiamethoxam effects on pea leaf weevil, Sitona lineatus. J. Insect Sci. 12:151. 10.1673/031.012.15101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzato E., Tufarelli V., Ceci E., Stellacci A. M., Laudadio V. (2012). Quality, yield and nitrogen fixation of faba bean seeds as affected by sulphur fertilization. Acta Agric. Scand. Sect. B: Soil Plant Sci. 62 732–738. 10.1080/09064710.2012.698642 [DOI] [Google Scholar]
- Chalk P. M. (1998). Dynamics of biologically fixed N in legume-cereal rotation: a review. Aust. J. Agric. Res. 49 303–316. 10.1071/A97013 [DOI] [Google Scholar]
- Chen W. (2009). Pollination, fertilization and floral traits co-segregating with autofertility in faba bean. J. New Seeds 10 14–30. 10.1080/15228860802594615 [DOI] [Google Scholar]
- Coda R., Varis J., Verni M., Rizzello C. G., Katina K. (2017). Improvement of the protein quality of wheat bread through faba bean sourdough addition. LWT - Food Sci. Technol. 82 296–302. 10.1016/j.lwt.2017.04.062 [DOI] [Google Scholar]
- Collado E., Artés-Hernández F., Aguayo E., Artés F., Fernández J., Gómez P. A. (2017). Quality changes of minimally processed fresh and microwave cooking of faba bean seeds. Actas Portug. Hort. 28 306–311. [Google Scholar]
- Cook R. T. A., Fox R. T. V. (1992). Erysiphe pisi var. pisi on faba beans and other legumes in Britain. Plant Pathol. 41 506–512. 10.1111/j.1365-3059.1992.tb02446.x [DOI] [Google Scholar]
- Crépon K., Marget P., Peyronnet C., Marget P., Peyronnet C., Carrouée B., et al. (2010). Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crop Res. 115 329–339. 10.1016/j.fcr.2009.09.016 [DOI] [Google Scholar]
- Cubero J. I. (1974). On the evolution of Vicia faba L. Theor. Appl Genet. 45 47–51. 10.1007/BF00283475 [DOI] [PubMed] [Google Scholar]
- Cubero J. I. (2017). “Leguminosas hortícolas: guisantes, judías y habas hortícolas” in Cultivos Hortícolas al Aire Libre, eds Maroto J.V., Baxauli C. (Almería: Cajamar Caja Rural; ), 703–741. [Google Scholar]
- Cunningham S. A., Le Feuvre D. (2013). Significant yield benefits from honeybee pollination of faba bean (Vicia faba) assessed at field scale. Field Crops Res. 149 269–275. 10.1016/j.fcr.2013.05.019 [DOI] [Google Scholar]
- Dane S., Laugale V., Lepse L., Silina D. (2017). Influence of legumes on soil fertility in strawberry-legume intercropping. Res. Rural Dev. 2 26–32. 10.22616/rrd.23.2017.045 [DOI] [Google Scholar]
- Davidson J. A., Kimber R. B. E. (2007). Integrated disease management of ascochyta blight in pulse crops. Eur. J. Plant Pathol. 119 99–110. 10.1007/s10658-007-9132-x [DOI] [Google Scholar]
- Davidson J. A., Pande S., Bretag T. W., Lindbeck K. D., Krishna-Kishore G. (2007). “Biology and management of Botrytis spp. in legume crops,” in Botrytis: Biology, Pathology and Control, eds Elad E., Williamson B.,, Tudzynski P., Delen N. (Berlin: Springer; ), 295–318. 10.1007/978-1-4020-2626-3_16 [DOI] [Google Scholar]
- del Pilar Cordovilla M., Ligero F., Lluch C. (1995). Influence of host genotypes on growth, symbiotic performance and nitrogen assimilation in faba bean (Vicia faba L.) under salt stress. Plant Soil 172 289–297. 10.1007/BF00011331 [DOI] [Google Scholar]
- del Pilar Cordovilla M., Ligero F., Lluch C. (1999). Effect of salinity on growth, nodulation and nitrogen assimilation in nodules of faba bean (Vicia faba L.). Appl. Soil Ecol. 11 1–7. 10.1016/S0929-1393(98)00132-2 [DOI] [Google Scholar]
- Duc G., Agrama H., Bao S., Berger J., Bourion V., De Ron A. M., et al. (2015b). Breeding annual grain legumes for sustainable agriculture: new methods to approach complex traits and target new cultivar ideotypes. Crit. Rev. Plant Sci. 34 381–411. 10.1080/07352689.2014.898469 [DOI] [Google Scholar]
- Duc G., Aleksić J. M., Marget P., Mikić A., Paull J., Redden R. J., et al. (2015a). “Faba bean,” in Grain Legumes, ed. De Ron A.M. (New York, NY: Springer; ), 141–178. 10.1007/978-1-4939-2797-5_5 [DOI] [Google Scholar]
- Duc G., Bao S., Baum M., Redden B., Sadiki M., Suso M. J., et al. (2010). Diversity maintenance and use of Vicia faba L. genetic resources. Field Crops Res. 115 270–278. 10.1016/j.fcr.2008.10.003 [DOI] [Google Scholar]
- Emeran A. A., Sillero J. C., Fernández-Aparicio M., Rubiales D. (2011). Chemical control of faba bean rust (Uromyces viciae-fabae). Crop Prot. 30 907–912. 10.1016/j.cropro.2011.02.004 [DOI] [Google Scholar]
- Etemadi F., Hashemi M., Shureshjani R., Autio W. R. (2017). Application of data envelopment analysis to assess performance efficiency of eight faba bean varieties. Agron. J. 109 1225–1231. 10.2134/agronj2016.10.0617 [DOI] [Google Scholar]
- European Commission (2017). Plant Variety Database, Available at: http://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases/search/public/index.cfm [Accessed June 6 2017]. [Google Scholar]
- Evenden M., Whitehouse C. M., Onge A. S., Vanderark L., Lafontaine J. P., Meers S., et al. (2016). Potential for semiochemical-based monitoring of the pea leaf weevil (Coleoptera: Curculionidae) on field pea (Fabaceae) in the Canadian. Prairie Provinces. Can. Entomol. 148 595–602. 10.4039/tce.2016.7 [DOI] [Google Scholar]
- FAO (2017). FAOSTAT Database. Food and Agriculture Organization of the United Nations. Available at: www.fao.org/faostat/ [accessed June 11 2017]. [Google Scholar]
- Fernández-Aparicio M., Shtaya M. J. Y., Emeran A. A., Allagui M. B., Kharrat M., Rubiales D. (2011). Effects of crop mixtures on chocolate spot development on faba bean grown in mediterranean climates. Crop Prot. 30 1015–1023. 10.1016/j.cropro.2011.03.016 [DOI] [Google Scholar]
- Fernández-Aparicio M., Sillero J. C., Rubiales D. (2007). Intercropping with cereals reduces infection by Orobanche crenata in legumes. Crop Prot. 26 1166–1172. 10.1016/j.cropro.2006.10.012 [DOI] [Google Scholar]
- Flores F., Hybl M., Knudsen J. C., Marget P., Muel F., Nadal S., et al. (2013). Adaptation of spring faba bean types across European climates. Field Crops Res. 145 1–9. 10.1016/j.fcr.2013.01.022 [DOI] [Google Scholar]
- Fouad M., Mohammed N., Aladdin H., Ahmed A., Xuxiao Z., Shiying B., et al. (2013). “5 – Faba Bean,” in Genetic and Genomic Resources of Grain Legume Improvement, eds Singh M., Upadhyaya H.D., Bisht I. S. (London: Elsevier; ), 113–136. 10.1016/B978-0-12-397935-3.00005-0 [DOI] [Google Scholar]
- Frenda A. S., Ruisi P., Saia S., Frangipane B., Di Miceli G., Amato G.,et al. (2013). The critical period of weed control in faba bean and chickpea in Mediterranean areas. Weed Sci. 61 452–459. 10.1614/WS-D-12-00137.1 [DOI] [Google Scholar]
- Gharibzahedi S. M. T., Mousavi S. M., Jafari S. M., Faraji K. (2012). Proximate composition, mineral content, and fatty acids profile of two varieties of lentil seeds cultivated in Iran. Chem. Nat. Compd. 47 976–978. 10.1007/s10600-012-0119-2 [DOI] [Google Scholar]
- Ghassemi-Golezani K., Ghanehpoor S., Mohammadi-Nasab D. (2009). Effects of water limitation on growth and grain filling of faba bean cultivars. J. Food Agric. Environ. 7 442–447. 10.1234/4.2009.2623 [DOI] [Google Scholar]
- Giambalvo D., Ruisi P., Saia S., Di Miceli G., Frenda A. S., Amato G. (2012). Faba bean grain yield, N2 fixation, and weed infestation in a long-term tillage experiment under rainfed Mediterranean conditions. Plant Soil 360 215–227. 10.1007/s11104-012-1224-5 [DOI] [Google Scholar]
- Girma F., Haile D. (2014). Effects of supplemental irrigation on physiological parameters and yield of faba bean (Vicia faba L.) varieties in the highlands of Bale, Ethiopia. J Agron. 13 29–34. 10.3923/ja.2014.29.34 [DOI] [Google Scholar]
- Göl ş., Doğanlar S., Frary A. (2017). Relationship between geographical origin, seed size and genetic diversity in faba bean (Vicia faba L.) as revealed by SSR markers. Mol. Genet. Genom. 292 991–999. 10.1007/s00438-017-1326-0 [DOI] [PubMed] [Google Scholar]
- Guoju X., Fengju Z., Juying H., Chengke L., Jing W., Fei M., et al. (2016). Response of bean cultures’ water use efficiency against climate warming in semiarid regions of China. Agric Water Manage. 173 84–90. 10.1016/j.agwat.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez N., Avila C. M., Moreno M. T., Torres A. M. (2008). Development of SCAR markers linked to zt-2 one of the genes controlling absence of tannins in faba bean. Aust. J. Agric. Res. 59 62–68. 10.1071/AR07019 [DOI] [Google Scholar]
- Habtegebriel B., Boydom A. (2016). Integrated management of faba bean black root rot (Fusarium solani) through varietal resistance, drainage and adjustment of planting time. J. Plant Pathol. Microbiol. 7:363. 10.4172/2157-7471.1000363 30035419 [DOI] [Google Scholar]
- Haidar M. A., Sidahmed M. M. (2000). Soil solarization and chicken manure for the control of Orobanche crenata and other weeds in Lebanon. Crop Prot. 19 169–173. 10.1016/S0261-2194(99)00083-6 [DOI] [Google Scholar]
- Hansen L. M., Lorentsen L., Boelt B. (2008). How to reduce the incidence of black bean aphids (Aphis fabae Scop.) attacking organic growing field beans (Vicia faba L.) by growing partially resistant bean varieties and by intercropping field beans with cereals. Acta Agric. Scand. Sec. B Soil Plant Sci. 58 359–364. 10.1080/09064710701788844 [DOI] [Google Scholar]
- Harlan J. R. (1975). Our vanishing genetic resources. Science 188 618–621. 10.1126/science.188.4188.617 [DOI] [PubMed] [Google Scholar]
- Hellal F. A., Abdelhamid M. T., Abo-Basha D. M., Zewainy R. M. (2012). Alleviation of the adverse effects of soil salinity stress by foliar application of silicon on Faba bean (Vicia faba L.). J. Appl. Sci. Res. 8 4428–4433. [Google Scholar]
- Hendawey M. H., Younes A. M. A. (2013). Biochemical evaluation of some faba bean cultivars under rainfed conditions at El-Sheikh Zuwayid. Ann. Agric. Sci. 58 183–193. 10.1016/j.aoas.2013.07.010 [DOI] [Google Scholar]
- Heuzé V., Tran G., Delagarde R., Lessire M., Lebas F. (2016). Faba bean (Vicia faba). Feedipedia, a Programme by INRA, CIRAD, AFZ and FAO. Available at: http://www.feedipedia.org/node/620 [Google Scholar]
- Huth N. I., Thorburn P. J., Radford B. J., Thornton C. M. (2010). Impacts of fertilisers and legumes on N2O and CO2 emissions from soils in subtropical agricultural systems: a simulation study. Agric. Ecosyst. Environ. 136 351–357. 10.1016/j.agee.2009.12.016 [DOI] [Google Scholar]
- Jensen E., Peoples M. B., Hauggaard-Nielsen H. (2010). Faba bean in cropping systems. Field Crops Res. 115 203–216. 10.1016/j.fcr.2009.10.008 [DOI] [Google Scholar]
- Jensen E., Peoples M. B., Boddey R. M., Gressho P. M., Hauggaard-Nielsen H., Alves B. J. R., et al. (2012). Legumes for mitigation of climate change and the provision of feedstock for biofuels and bioreneries. A review. Agron. Sustain. Dev. 32 329–364. 10.1007/s13593-011-0056-7 [DOI] [Google Scholar]
- Jezierny D., Mosenthin R., Sauer N., Schwadorf K., Rosenfelder-Kuon P. (2017). Methodological impact of starch determination on starch content and ileal digestibility of starch in grain legumes for growing pigs. J. Anim. Sci. Biotechnol. 8:4 10.1186/s40104-016-0131-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilani M., Daneshian J., Rabiee M. (2012). Effect of pre-harvest desiccation of paraquat on grain yield and some agronomic characteristics of Faba bean. Adv. Environ. Biol. 6 2502–2504. [Google Scholar]
- Kalburtji K. L., Mamolos A. P. (2001). Competition between Canada thistle [Cirsium arvense (L.) Scop.] and faba bean (Vicia faba L.). J. Agron. Crop Sci. 186 261–265. 10.1046/j.1439-037x.2001.00484.x [DOI] [Google Scholar]
- Karaköy T., Baloch F. S., Toklu F., Özkan H. (2014). Variation for selected morphological and quality-related traits among 178 faba bean landraces collected from Turkey. Plant Genetic Res. 12 5–13. 10.1017/S1479262113000208 [DOI] [Google Scholar]
- Karkanis A., Ntatsi G., Kontopoulou C. K., Pristeri A., Bilalis D., Savvas D. (2016a). Field pea in european cropping systems: adaptability, biological nitrogen fixation and cultivation practices. Not. Bot. Horti Agrobot. Cluj Napoca. 44 325–336. 10.15835/nbha44210618 [DOI] [Google Scholar]
- Karkanis A., Travlos I. S., Bilalis D., Tabaxi E. I. (2016b). “Integrated weed management in winter cereals in Southern Europe,” in Weed and Pest Control: Molecular Biology, Practices and Environmental Impact, eds Travlos I. S., Bilalis D. J., Chachalis D. (New York, NY: Nova Science Publishers, Inc.), 1–15. [Google Scholar]
- Katerji N., Mastrorilli M., Lahmer F. Z., Maalouf F., Oweis T. (2011). Faba bean productivity in saline-drought conditions. Eur. J. Agron. 35 2–12 10.1016/j.eja.2011.03.001 [DOI] [Google Scholar]
- Keneni G., Bekele E., Getu E., Imtiaz M., Damte T., Mulatu B., et al. (2011). Breeding food legumes for resistance to storage insect pests: potential and limitations. Sustain 3 1399–1415. 10.3390/su3091399 [DOI] [Google Scholar]
- Khamassi K., Ben Jeddi F., Hobbs D., Irigoyen J., Stoddard F., O’sullivan D. M., et al. (2013b). A baseline study of vicine-convicine levels in faba bean (Vicia faba L.) germplasm. Plant Genetic Res. 11 250–257. 10.1017/S1479262113000105 [DOI] [Google Scholar]
- Khamassi K., Harbaoui K., Teixeira Da Silva J. A., Jeddi F. B. (2013a). Optimal germination temperature assessed by indices and models in Field bean (Vicia faba L. var. minor). Agric. Conspec. Sci. 78 131–136. [Google Scholar]
- Khan H. R., Paull J. G., Siddique K. H. M., Stoddard F. L. (2010). Faba bean breeding for drought-affected environments: a physiological and agronomic perspective. Field Crops Res. 115 279–286. 10.1016/j.fcr.2009.09.003 [DOI] [Google Scholar]
- Khazaei H., Street K., Santanen A., Bari A., Stoddard F. L. (2013). Do faba bean (Vicia faba L.) accessions from environments with contrasting seasonal moisture availabilities differ in stomatal characteristics and related traits? Genet. Resour. Crop. Evol. 60 2343–2357. 10.1007/s10722-013-0002-4 [DOI] [Google Scholar]
- Knott C. M. (1999). Irrigation of spring field beans (Vicia faba): response to timing at different crop growth stages. J. Agric. Sci. 132 407–415. 10.1017/S0021859699006541 [DOI] [Google Scholar]
- Koivunen E., Partanen K., Perttilä S., Palander S., Tuunainen P., Valaja J. (2016). Digestibility and energy value of pea (Pisum sativum L.), faba bean (Vicia faba L.) and blue lupin (narrow-leaf) (Lupinus angustifolius) seeds in broilers. Anim. Feed Sci. Technol. 218 120–127. 10.1016/j.anifeedsci.2016.05.007 [DOI] [Google Scholar]
- Köpke U., Nemecek T. (2010). Ecological services of faba bean. Field Crops Res. 115 217–233. 10.1016/j.fcr.2009.10.012 [DOI] [Google Scholar]
- Landry E. J., Fuchs S. J., Hu J. (2016). Carbohydrate composition of mature and immature faba bean seeds. J. Food Compos. Anal. 50 55–60. 10.1016/j.jfca.2016.05.010 [DOI] [Google Scholar]
- Lepse L., Dane S., Zeipiņa S., Domínguez-Perles R., Rosa E. A. (2017). Evaluation of vegetable-faba bean (Vicia faba L.) intercropping under Latvian agro-ecological conditions. J. Sci. Food Agric. 97 4334–4342. 10.1002/jsfa.8239 [DOI] [PubMed] [Google Scholar]
- Lestingi A., Bovera F., de Giorgio D., Ventrella D., Tateo A. (2011). Effect of tillage system on seed yield, chemical composition and nutritive value of horse bean (Vicia faba L. Minor) grown under Mediterranean conditions. J. Food Agric. Environ. 9 228–235. [Google Scholar]
- Li C., Ganjyal G. M. (2017). Chemical composition, pasting, and thermal properties of 22 different varieties of peas and lentils. Cereal Chem. 94 392–399. 10.1094/CCHEM-04-16-0080-R [DOI] [Google Scholar]
- Link W., Balko C., Stoddard F. L. (2010). Winter hardiness in faba bean: physiology and breeding. Field Crops Res. 115 287–296. 10.1016/j.fcr.2008.08.004 [DOI] [Google Scholar]
- Lithourgidis A. S., Tzavella-Klonari K., Roupakias D. G. (2003). The causal fungus of stem rot disease of faba beans in Greece. J Phytopathol. 151 631–635. 10.1046/j.0931-1785.2003.00778.x [DOI] [Google Scholar]
- Lizarazo C. I., Lampi A. M., Sontag-Strohm T., Liu J., Piironen V., Stoddard F. L. (2015). Nutritive quality and protein production from grain legumes in a boreal climate. J. Sci. Food Agric. 95 2053–2064. 10.1002/jsfa.6920 [DOI] [PubMed] [Google Scholar]
- Longobardi F., Sacco D., Casiello G., Ventrella A., Sacco A. (2015). Chemical profile of the carpino broad bean by conventional and innovative physicochemical analyses. J. Food Qual. 38 273–284. 10.1111/jfq.12143 [DOI] [Google Scholar]
- López-Bellido R. J., López-Bellido L., López-Bellido F. J., Castillo J. E. (2003). Faba bean (Vicia faba L.) response to tillage and soil residual nitrogen in a continuous rotation with wheat (Triticum aestivum L.) under rainfed Mediterranean conditions. Agron. J. 95 1253–1261. 10.2134/agronj2003.1253 [DOI] [Google Scholar]
- Loss S. P., Siddique K. H. M. (1997). Adaptation of faba bean (Vicia faba L.) to dryland mediterranean-type environments. I. Seed yield and yield components. Field Crops Res. 52 17–28. 10.1016/S0378-4290(96)03455-7 [DOI] [Google Scholar]
- Luo Y-W., Xie W-H. (2013). Effect of different processing methods on certain antinutritional factors and protein digestibility in green and white faba bean (Vicia faba L.). CYTA- Food. 11 43–49. 10.1080/19476337.2012.681705 [DOI] [Google Scholar]
- Maalouf F., Nachit M., Ghanem M. E., Singh M. (2015). Evaluation of faba bean breeding lines for spectral indices, yield traits and yield stability under diverse environments. Crop Pasture Sci. 66 1012–1023. 10.1071/CP14226 [DOI] [Google Scholar]
- Mahmoudi M., Sahragard A., Sendi J. J. (2010). Foraging efficiency of Lysiphlebus fabarum marshall (Hymenoptera: Aphidiidae) parasitizing the black bean aphid, Aphis fabae Scopoli (Hemiptera: Aphididae), under laboratory conditions. J. Asia-Pac. Entomol. 13 111–116. 10.1016/j.aspen.2009.11.007 [DOI] [Google Scholar]
- Mandal U. K., Singh G., Victor U. S., Sharma K. L. (2003). Green manuring: its effect on soil properties and crop growth under rice-wheat cropping system. Eur. J. Agron. 19 225–237. 10.1016/S1161-0301(02)00037-0 [DOI] [Google Scholar]
- Maqbool A., Shafiq S., Lake L. (2010). Radiant frost tolerance in pulse crops-a review. Euphytica 172 1–12. 10.1007/s10681-009-0031-4 [DOI] [Google Scholar]
- Marcellos H., Moore K. J., Nikandrow A. (1995). Influence of foliar-applied fungicides on seed yield of faba bean (Vicia faba L.) in Northern New South Wales. Aust. J. Exp. Agric. 35 97–102. 10.1071/EA9950097 [DOI] [Google Scholar]
- Martos-Fuentes M. (2017). Genotyping, phenotyping and transcriptomic analysis of accessions of Vicia faba, Pisum sativum and Vigna unguiculata. Ph.D. Thesis, Technical University of Cartagena, Spain. [Google Scholar]
- Mauromicale G., Restuccia G., Marchese M. (2001). Soil solarization, a non-chemical technique for controlling Orobanche crenata and improving yield of faba bean. Agronomy 21 757–765. 10.1051/agro:2001167 [DOI] [Google Scholar]
- Mbazia A., Omri B., Youssef N., Kharrat M. (2016). Tunisian isolates of Trichoderma spp. and Bacillus subtilis can control Botrytis fabae on faba bean. Biocontrol Sci. Technol. 26 915–927. 10.1080/09583157.2016.1168775 [DOI] [Google Scholar]
- McDonald M., Copeland L. O. (1997). Seed Production: Principles and Practices. New York, Springer: 1–749. 10.1007/978-1-4615-4074-8 [DOI] [Google Scholar]
- McNaughton K. E., Blackshaw R. E., Waddell K. A., Gulden R. H., Sikkema P. H., Gillard C. L. (2015). Effect of five desiccants applied alone and in combination with glyphosate in dry edible bean (Phaseolus vulgaris L.). Can. J. Plant Sci. 95 1235–1242. 10.4141/cjps-2015-098 [DOI] [Google Scholar]
- Metwali E. M. R., Abdelmoneim T. S., Bakheit M. A., Kadasa N. M. S. (2015). Alleviation of salinity stress in faba bean (Vicia faba L.) plants by inoculation with plant growth promoting rhizobacteria (PGPR). Plant Omics 8 449–460. [Google Scholar]
- Migdadi H. M., El-Harty E. H., Salamh A., Khan M. A. (2016). Yield and proline content of faba bean genotypes under water stress treatments. J. Anim. Plant Sci. 26 1772–1779. 10.3390/ijms160510214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. R., Lighthiser E. J., Jones C. A., Holmes J. A., Rick T. L., Wraith J. M. (2011). Pea green manure management affects organic winter wheat yield and quality in semiarid Montana. Can. J. Plant Sci. 91 497–508. 10.4141/cjps10109 [DOI] [Google Scholar]
- Mohamed S. S. E., Babiker H. M. (2012). Effects of Rhizobium inoculation and urea fertilization on faba bean (Vicia faba L.) production in a semi-desert zone. Adv. Environ. Biol. 6 824–830. [Google Scholar]
- Mona A. M., Sabah M. A., Rehab A. M. (2011). Influence of potassium sulfate on faba bean yield and quality. Aust. J. Basic Appl. Sci. 5 87–95. [Google Scholar]
- Moorthi R. N., Armstrong C. L. H., Janda K., Ponsler-Sipes K., Asplin J. R., Moe S. M. (2015). The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am. J. Nephrol. 40 582–591. 10.1159/000371498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M., Martinez A. (1980). The divided world of V. faba. FABIS Newslett. 2 18–19. [Google Scholar]
- Moussart A., Even M. N., Tivoli B. (2008). Reaction of genotypes from several species of grain and forage legumes to infection with a French pea isolate of the oomycete Aphanomyces euteiches. Eur. J. Plant Pathol. 122 321–333. 10.1007/s10658-008-9297-y [DOI] [Google Scholar]
- Multari S., Stewart D., Russell W. R. (2015). Potential of fava bean as future protein supply to partially replace meat intake in the human diet. Compr. Rev. Food Sci. Food Saf. 14 511–522. 10.1111/1541-4337.12146 [DOI] [Google Scholar]
- Muñoz-Romero V., López-Bellido L., López-Bellido R. J. (2011). Faba bean root growth in a Vertisol: tillage effects. Field Crops Res. 120 338–344. 10.1016/j.fcr.2010.11.008 [DOI] [Google Scholar]
- Mwanamwenge J., Loss S., Siddique K., Cocks P. (1999). Effect of water stress during floral initiation, flowering and podding on the growth and yield of faba bean (Vicia faba L.). Eur. J. Agron. 11 1–11. 10.1016/S1161-0301(99)00003-9 [DOI] [Google Scholar]
- Nasto T., Sallaku G., Balliu A. (2016). Phenotypic diversity among faba bean (Vicia faba) local genotypes in Albania. Acta Hortic. 1142 233–238. 10.17660/ActaHortic.2016.1142.36 [DOI] [Google Scholar]
- Nebiyu A., Diels J., Boeckx P. (2016). Phosphorus use efficiency of improved faba bean (Vicia faba) varieties in low-input agro-ecosystems. J. Plant Nutr. Soil Sci. 179 347–354. 10.1002/jpln.201500488 [DOI] [Google Scholar]
- Neme K., Bultosa G., Bussa N. (2015). Nutrient and functional properties of composite flours processed from pregelatinised barley, sprouted faba bean and carrot flours. Int. J. Food Sci. Technol. 50 2375–2382. 10.1111/ijfs.12903 [DOI] [Google Scholar]
- Neugschwandtner R., Ziegler K., Kriegner S., Wagentristl H., Kaul H. P. (2015). Nitrogen yield and nitrogen fixation of winter faba beans. Acta Agric. Scand. Sect. B Soil Plant Sci. 65 658–666. 10.1080/09064710.2015.1042028 [DOI] [Google Scholar]
- Niewiadomska A., Barłóg P., Borowiak K., Wolna-Maruwka A. (2015). The effect of sulphur and potassium fertilisation on the nitrogenase and microbial activity in soil under broad bean (Vicia faba L.) cultivation. Fresenius Environ. Bull. 24 723–732. [Google Scholar]
- Nozzolillo C., Ricciardi L., Lattanzio V. (1989). Flavonoid constituents of seed coats of Vicia faba (Fabaceae) in relation to genetic control of their color. Can. J. Bot. 67 1600–1604. 10.1139/b89-200 [DOI] [Google Scholar]
- Ntatsi G., Karkanis A., Yfantopoulos D., Olle M., Travlos I., Thanopoulos R., Bilalis D., et al. (2018). Impact of variety and farming practices on growth, yield, weed flora and symbiotic nitrogen fixation in faba bean cultivated for fresh seed production. Acta Agric. Scand. Sec. B Plant Soil Sci. 38 619–630. 10.1080/09064710.2018.1452286 [DOI] [Google Scholar]
- Nuessly G. S., Hentz M. G., Beiriger R., Scully B. T. (2004). Insects associated with faba bean, Vicia faba (Fabales: Fabaceae), in southern Florida. Fla. Entomol. 87 204–211. 10.1653/0015-4040(2004)087[0204:IAWFBV]2.0.CO;2 [DOI] [Google Scholar]
- O’Donovan J. T., Grant C. A., Blackshaw R. E., Harker K. N., Johnson E. N., Gan Y. T., et al. (2014). Rotational effects of legumes and non-legumes on hybrid canola and malting. Agron. J. 106 1921–1932. 10.2134/agronj14.0236 [DOI] [Google Scholar]
- Oliveira H. R., Tomás D., Silva M., Lopes S., Viegas W., Veloso M. M. (2016). Genetic diversity and population structure in Vicia faba L. landraces and wild related species assessed by nuclear SSRs. PLoS One 11:e0154801. 10.1371/journal.pone.0154801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omri Benyoussef N., Le May C., Mlayeh O., Kharrat M. (2012). First report of Didymella fabae, teleomorph of Ascochyta fabae, on faba bean crop debris in Tunisia. Phytopathol. Mediter. 51 369–373. [Google Scholar]
- Ortiz V., Navarro E., Castro S., Carazo G., Romero J. (2006). Incidence and transmission of Faba bean necrotic yellows virus (FBNYV) in Spain. Span. J. Agric. Res. 4 255–260. 10.5424/sjar/2006043-200 [DOI] [Google Scholar]
- Oweis T., Hachum A., Pala M. (2005). Faba bean productivity under rainfed and supplemental irrigation in northern Syria. Agric. Water Manag. 73 57–72. 10.1016/j.agwat.2004.09.022 [DOI] [Google Scholar]
- Pampana S., Masoni A., Arduini I. (2016). Response of cool-season grain legumes to waterlogging at flowering. Can. J. Plant Sci. 96 597–603. 10.1139/cjps-2015-0268 [DOI] [Google Scholar]
- Patrick J. W., Stoddard F. L. (2010). Physiology of flowering and grain filling in faba bean. Field Crops Res. 115 234–242. 10.1016/j.fcr.2009.06.005 [DOI] [Google Scholar]
- Patterson C. A., Curran J., Der T. (2017). Effect of processing on antinutrient compounds in pulses. Cereal Chem. 94 2–10. 10.1094/CCHEM-05-16-0144-FI [DOI] [Google Scholar]
- Pérez-de-Luque A., Eizenberg H., Grenz J. H., Sillero J. C., Ávila C., et al. (2010). Broomrape management in faba bean. Field Crops Res. 115 319–328. 10.1016/j.fcr.2009.02.013 [DOI] [Google Scholar]
- Pérez-de-Luque A., Flores F., Rubiales D. (2016). Differences in crenate broomrape parasitism dynamics on three legume crops using a thermal time model. Front. Plant Sci. 7:1910. 10.3389/fpls.2016.01910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-de-Luque A., Sillero J. C., Moral A., Cubero J. I., Rubiales D. (2004). Effect of sowing date and host resistance on the establishment and development of Orobanche crenata in faba bean and common vetch. Weed Res. 44 282–288. 10.1111/j.1365-3180.2004.00401.x [DOI] [Google Scholar]
- Pietrzak W., Kawa-Rygielska J., Król B., Lennartsson P. R., Taherzadeh M. J. (2016). Ethanol, feed components and fungal biomass production from field bean (Vicia faba var. equina) seeds in an integrated process. Bioresour. Technol. 216 69–76. 10.1016/j.biortech.2016.05.055 [DOI] [PubMed] [Google Scholar]
- Pociecha E., Kościelniak J., Filek W. (2008). Effects of root flooding and stage of development on the growth and photosynthesis of field bean (Vicia faba L. minor). Acta Physiol. Plant. 30 529–535. 10.1007/s11738-008-0151-9 [DOI] [Google Scholar]
- Purhematy A., Ahmadi K., Moshrefi M. (2013). Toxicity of thiacloprid and fenvalerate on the black bean aphid, Aphis fabae, and biosafety against its parasitoid, Lysiphlebus fabarum. J. Biopestic. 6 207–210. [Google Scholar]
- Ray H., Bett K., Tar’an B., Vandenberg A., Thavarajah D., Warkentin T. (2014). Mineral micronutrient content of cultivars of field pea, chickpea, common bean, and lentil grown in Saskatchewan, Can. Crop Sci. 54 1698–1708. 10.2135/cropsci2013.08.0568 [DOI] [Google Scholar]
- Rubiales D., Avila C. M., Sillero J. C., Hybl M., Narits L., Sass O., et al. (2012). Identification and multi-environment validation of resistance to Ascochyta fabae in faba bean (Vicia faba). Field Crops Res. 126 165–170. 10.1016/j.fcr.2011.10.012 [DOI] [Google Scholar]
- Rubiales D., Flores F., Emeran A. A., Kharrat M., Amri M., Rojas-Molina M. M., et al. (2014). Identification and multi-environment validation of resistance against broomrapes (Orobanche crenata and Orobanche foetida) in faba bean (Vicia faba). Field Crops Res. 166 58–65. 10.1016/j.fcr.2014.06.010 [DOI] [Google Scholar]
- Sahile S., Fininsa C., Sakhuja P. K., Ahmed S. (2008). Effect of mixed cropping and fungicides on chocolate spot (Botrytis fabae) of faba bean (Vicia faba) in Eth. Crop Prot. 27 275–282. 10.1016/j.cropro.2007.06.003 [DOI] [Google Scholar]
- Sahile S., Fininsa C., Sakhuja P. K., Ahmed S. (2010). Yield loss of faba bean (Vicia faba) due to chocolate spot (Botrytis fabae) in sole and mixed cropping systems in Ethiopia. Arch. Phytopathol. Plant Prot. 43 1144–1159. 10.1080/03235400802343791 [DOI] [Google Scholar]
- Salahin N., Alam M. D. K., Islam M. D. M., Naher L., Majid N. M. (2013). Effects of green manure crops and tillage practice on maize and rice yields and soil properties. Aust. J. Crop Sci. 7 1901–1911. [Google Scholar]
- Sallam A., Arbaoui M., El-Esawi M., Abshire N., Martsch R. (2016). Identification and verification of QTL associated with frost tolerance using linkage mapping and GWAS in winter faba bean. Front. Plant Sci. 7:1098. 10.3389/fpls.2016.01098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam A., Ghanbari M., Martsch R. (2017). Genetic analysis of winter hardiness and effect of sowing date on yield traits in winter faba bean. Sci. Hortic. 224 296–301. 10.1016/j.scienta.2017.04.015 [DOI] [Google Scholar]
- Sallam A., Martsch R., Moursi Y. S. (2015). Genetic variation in morpho-physiological traits associated with frost tolerance in faba bean (Vicia faba L.). Euphytica 205 395–408. 10.1007/s10681-015-1395-2 [DOI] [Google Scholar]
- Sangakkara U. R., Hartwig U. A., Nösberger J. (1996). Soil moisture and potassium affect the performance of symbiotic nitrogen fixation in faba bean and common bean. Plant Soil 184 123–130. 10.1007/BF00029282 [DOI] [Google Scholar]
- Schubert E., Mengel K., Schubert S. (1990). Soil pH and calcium effect on nitrogen fixation and growth of broad bean. Agron. J. 82 967–969. 10.2134/agronj1990.00021962008200050026x [DOI] [Google Scholar]
- Seidenglanz M.,, Huņady I. (2016.) Effects of faba bean (Vicia faba) varieties on the development of Bruchus rufimanus. Czech J. Genet. Plant Breed. 52 22–29. 10.17221/122/2015-CJGPB [DOI] [Google Scholar]
- Sharifi P. (2014). Correlation and path coefficient analysis of yield and yield component in some of broad bean (Vicia faba L.) genotypes. Genet. Belgrad. 469 905–914. 10.2298/GENSR1403905S [DOI] [Google Scholar]
- Shi L., Mu K., Arntfield S. D., Nickerson M. T. (2017). Changes in levels of enzyme inhibitors during soaking and cooking for pulses available in Canada. J. Food Sci. Technol. 54 1014–1022. 10.1007/s13197-017-2519-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiran B., Kiani S., Sehgal D., Hafizi A., Chaudhary M., Raina S. N. (2014). Internal transcribed spacer sequences of nuclear ribosomal DNA resolving complex taxonomic history in the genus Vicia L. Genet. Resour. Crop Evol. 61 909–925. 10.1007/s10722-014-0086-5 [DOI] [Google Scholar]
- Shiying B., Xiaoming W., Zhendong Z., Xuxiao Z., Kumari S., Freeman A., et al. (2007). Survey of faba bean and field pea viruses in Yunnan Province, China. Austr. Plant Pathol. 36 347–353. 10.1071/AP07034 [DOI] [Google Scholar]
- Siddique K. H. M., Loss S. P. (1999). Studies on sowing depth for chickpea (Cicer arietinum L.), faba bean (Vicia faba L.) and Lentil (Lens culinaris Medik) in a Mediterranean-type environment of south-western Australia. J. Agron. Crop Sci. 182 105–112. 10.1046/j.1439-037x.1999.00281.x [DOI] [Google Scholar]
- Siddique K. H. M., Erskine W., Hobson K., Knights E. J., Leonforte A., Khan T. N., et al. (2013). Cool-season grain legume improvement in Australia-use of genetic resources. Crop Pasture Sci. 64 347–360. 10.1071/CP13071 [DOI] [Google Scholar]
- Siddiqui M. H., Al-Khaishany M. Y., Al-Qutami M. A., Al-Whaibi M. H., Grover A., Ali H. M., et al. (2015). Response of different genotypes of faba bean plant to drought stress. Int. J. Mol. Sci. 16 10214–10227. 10.3390/ijms160510214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillero J. C., Villegas-Fernandez A. M., Thomas J., Rojas-Molina M. M., Emeran A. A., Fernandez-Aparicio M., et al. (2010). Faba bean breeding for disease resistance. Field Crop Res. 115 297–307. 10.1016/j.fcr.2009.09.012 [DOI] [Google Scholar]
- Sincik M., Turan Z. M., Göksoy A. T. (2008). Responses of potato (Solanum tuberosum L.) to green manure cover crops and nitrogen fertilization rates. Am. J. Pot. Res. 85 150–158. 10.1007/s12230-008-9011-9 [DOI] [Google Scholar]
- Singh A. K., Bhatt B. P., Upadhyaya A., Kumar S., Sundaram P. K., Singh B. K., et al. (2012). Improvement of faba bean (Vicia faba L.) yield and quality through biotechnological approach: a review. Afr. J. Biotechnol. 11 15264–15271 [Google Scholar]
- Solaiman Z., Colmer T. D., Loss S. P., Thomson B. D., Siddique K. H. M. (2007). Growth responses of cool-season grain legumes to transient waterlogging. Aust. J. Agric. Res. 58 406–41. 10.1071/AR06330 [DOI] [Google Scholar]
- Stoddard F. L. (1991). Pollen vectors and pollination of faba beans in southern Australia. Aust. J. Agric. Res. 42 1173–1178. 10.1071/AR9911173 [DOI] [Google Scholar]
- Stoddard F. L., Balko C., Erskine W., Khan H. R., Link W., Sarker A. (2006). Screening techniques and sources of resistance to abiotic stresses in cool-season food legumes. Euphytica 147 167–186. 10.1007/s10681-006-4723-8 [DOI] [Google Scholar]
- Stoddard F. L., Nicholas A. H., Rubiales D., Thomas J., Villegas-Fernández A. M. (2010). Integrated pest management in faba bean. Field Crops Res. 115 308–318. 10.1016/j.fcr.2009.07.002 [DOI] [Google Scholar]
- Suso M., Moreno M., Mondragao-Rodrigues F., Cubero J. (1996). Reproductive biology of Vicia faba: role of pollination conditions. Field Crops Res. 46 81–91. 10.1016/0378-4290(95)00089-5 [DOI] [Google Scholar]
- Tavakkoli E., Paull J., Rengasamy P., McDonald G. K. (2012). Comparing genotypic variation in faba bean (Vicia faba L.) in response to salinity in hydroponic and field experiments. Field Crops Res. 127 99–108. 10.1016/j.fcr.2011.10.016 [DOI] [Google Scholar]
- Tawaha A. M., Turk M. A. (2001). Crop-weed competition studies in faba bean (Vicia faba L.) under rainfed conditions. Acta Agron. Hung. 49 299–303. 10.1556/AAgr.49.2001.3.11 [DOI] [Google Scholar]
- Temesgen T., Keneni G., Sefera T., Jarso M. (2015). Yield stability and relationships among stability parameters in faba bean (Vicia faba L.) genotypes. Crop J. 3 258–268. 10.1016/j.cj.2015.03.004 [DOI] [Google Scholar]
- Terzopoulos P. J., Bebeli P. J. (2008). Genetic diversity analysis of Mediterranean faba bean (Vicia faba L.) with ISSR markers. Field Crops Res. 108 39–44. 10.1016/j.fcr.2008.02.015 [DOI] [Google Scholar]
- Thomas K., Thanopoulos R., Knüpffer H., Bebeli P. J. (2013). Plant genetic resources in a touristic island: the case of lefkada (Ionian Islands, Greece). Genet. Resour. Crop Evol. 60 2431–2455. 10.1007/s10722-013-0011-3 [DOI] [Google Scholar]
- Torres A. M., Román B., Avila C. M., Satovic Z., Rubiales D., Sillero J. C., et al. (2006). Faba bean breeding for resistance against biotic stresses: towards application of marker technology. Euphytica 147 67–80. 10.1007/s10681-006-4057-6 [DOI] [Google Scholar]
- Turco I., Ferretti G., Bacchetti T. (2016). Review of the health benefits of faba bean (Vicia faba L.) polyphenols. J. Food Nutr. Res. 55 283–293. [Google Scholar]
- van Leur J. A. G., Southwell R. J., Mackie J. M. (2008). Aphanomyces root rot on faba bean in northern NSW. Australas. Plant Dis. Notes 3 8–9. 10.1071/DN08004 [DOI] [Google Scholar]
- Villegas-Fernández A. M., Sillero J. C., Rubiales D. (2012). Screening faba bean for chocolate spot resistance: evaluation methods and effects of age of host tissue and temperature. Eur. J. Plant Pathol. 132 443–453. 10.1007/s10658-011-9889-9 [DOI] [Google Scholar]
- Witten S., Böhm H., Aulrich K. (2015). Effect of variety and environment on the contents of crude nutrients, lysine, methionine and cysteine in organically produced field peas (Pisum sativum L) and field beans (Vicia faba L). Landbauforsch Volkenrode 65 205–216. [Google Scholar]
- Woyengo T. A., Nyachoti C. M. (2012). IIeal digestibility of amino acids for zero-tannin faba bean (Vicia faba L.) fed to broiler chicks. Poult. Sci. 91 439–443. 10.3382/ps.2011-01678 [DOI] [PubMed] [Google Scholar]
- Youseif S. H., Fayrouz H. A. E.-M., Saleh S. A. (2017). Improvement of faba bean yield using Rhizobium/agrobacterium inoculant in low-fertility sandy soil. Agronomy 7:2 10.3390/agronomy7010002 [DOI] [Google Scholar]
- Yucel D. O. (2013). Optimal intra-row spacing for production of local faba bean (Vicia faba L. major) cultivars in the Mediterranean conditions. Pak. J. Bot. 45 1933–1938. [Google Scholar]
- Zeid M., Schön C. C., Link W. (2003). Genetic diversity in recent elite faba bean lines using AFLP markers. Theor. Appl. Genet. 107 1304–1314. 10.1007/s00122-003-1350-9 [DOI] [PubMed] [Google Scholar]
- Zhao J., Sykacek P., Bodner G., Rewald B. (2018). Root traits of European Vicia faba cultivars – Using machine learning to explore adaptations to agroclimatic conditions. Plant Cell Environ. 10.1111/pce.13062 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Zhou R., Hyldgaard B., Yu X., Rosenqvist E., Ugarte R. M., Yu S.,et al. (2018). Phenotyping of faba beans (Vicia faba L.) under cold and heat stresses using chlorophyll fluorescence. Euphytica 214:68 10.1007/s10681-018-2154-y [DOI] [Google Scholar]
- Zong X., Liu X., Guan J., Wang S., Liu Q., Paull J. G., et al. (2009). Molecular variation among Chinese and global winter faba bean germplasm. Theor. Appl. Genet. 118 971–978. 10.1007/s00122-008-0954-5 [DOI] [PubMed] [Google Scholar]
- Zong X., Ren J., Guan J., Wang S., Liu Q., Paull J., Redden R. (2010). Molecular variation among Chinese and global germplasm in spring faba bean areas. Plant Breed. 129 508–513. 10.1111/j.1439-0523.2009.01718.x [DOI] [Google Scholar]
- Zou L., Yli-Halla M., Stoddard F. L., Mäkelä P. S. A. (2015). Effects of break crops on yield and grain protein concentration of barley in a boreal climate. PLoS One 10:e0130765. 10.1371/journal.pone.013076 [DOI] [PMC free article] [PubMed] [Google Scholar]