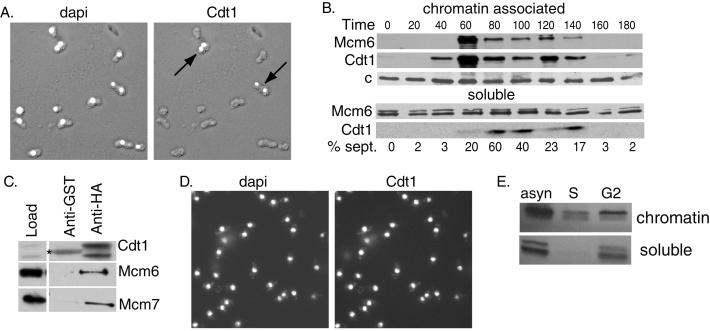
Figure 1.
Regulation of Cdt1 expression in the cell cycle. (A) Cells expressing Cdt1-HA under the control of its endogenous promoter were fixed and stained with 4′,6-diamidino-2-phenylindole (dapi, left) or 16B12 anti-HA antibodies (right). The stained images are superimposed on Nomarski images of the fixed cells. (B) cdc25-22 cells were arrested in G2 by shifting to the nonpermissive temperature (35.5°C) for 4 h and released into synchronous culture at the permissive temperature (25°C). Cell extracts were prepared at the indicated times and fractionated into chromatin and soluble fractions. The Mcm6 and Cdt1 proteins were detected by Western blotting. A nonspecific band detected with anti-Mcm6 antibody (c) served as a loading control. (C) Extracts prepared from cells expressing Cdt1-HA under the control of the nmt1 promoter (pREP41X vector) were incubated with anti-HA antibody or nonspecific anti-glutathione S-transferase (anti-GST) antibody on agarose beads. The immunoprecipitates were analyzed by Western blotting to detect Cdt1, Mcm6, or Mcm7 proteins. A 5-fold excess of the immunoprecipitate was loaded compared with the starting material. The band marked with an asterisk is Ig heavy chain. (D) Cells expressing Cdt1-HA under the control of the 41X-nmt1 promoter were fixed and stained with 4′,6-diamidino-2-phenylindole (left) or anti-HA antibodies (right). (E) cdc25-22 cells expressing Cdt1 under the control of the 41X-nmt1 promoter were arrested in S phase (S) by incubation for 4 h in hydroxyurea (25 mM) or in G2 phase by incubation for 4 h at the nonpermissive temperature. Extracts prepared from the arrested cells and from a control population of asynchronous (asyn) cells were fractionated into detergent insoluble (chromatin) and soluble fractions. Cdt1 was detected by Western blotting with anti-Cdt1 antibodies.