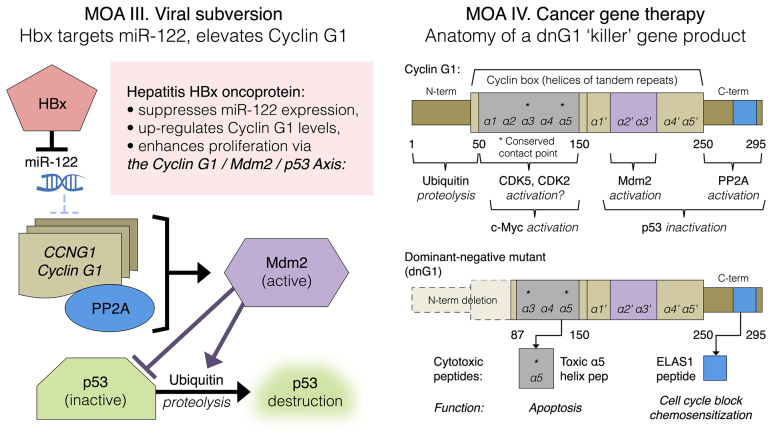
Figure 9.
Left panel: Mechanism-of-action (MOA) III. HBx-mediated viral subversion of the hepatocellular division cycle operates by suppression of miR-122, hence de-repression of CCNG1 expression. Right panel: MOA IV. Cancer gene therapy, dng1 structure. The cytocidal dnG1 protein, a dominant-negative mutant construct of cyclin G1, is devoid of the ‘ubiquitinated’ N-terminus (proteolytic processing), as well as the first two helical segments (α1 and α2) of the definitive cyclin box, characteristically arrayed in cyclins as a tandem set of helical segments, including two highly-conserved residues (asterisks) essential for cyclin-dependent kinase (CDK) binding. The cytocidal dnG1 protein, which induces apoptosis in proliferative cells, retains the presumptive (?) CDK contact points (Helix α3*, α5*) and the structural domains attributed to PP2A, β' and Mdm2 binding. Remarkably, new therapeutic peptides (e.g., ELAS1 and α5 Helix peptides) derived from structures or homologous interfaces contained within the dnG1 protein are reported to induce cell cycle blockade and apoptosis, respectively.