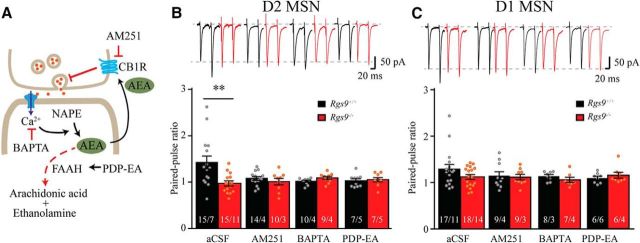
Figure 6.
Involvement of RGS9–2 in regulation of presynaptic glutamate release via modulation of eCB signaling. A, Schematic of the assay design to study possible signaling pathways involved in retrograde synaptic signaling that regulates presynaptic vesicle release in MSN. NAPE, N-arachidonoyl phosphatidylethanolamine. B, Representative traces and summarized bar graph show that genetic deletion of RGS9–2 significantly reduced PPR in D2-MSNs (aCSF; t(28) = 3.0, p = 0.005), whereas application of CB1 receptor blocker AM251, calcium chelator BAPTA, or FAAH activator PDP-EA mimicked the effect of RGS9–2 ablation. No significant differences between genotypes were observed in the presence of these drugs (t(21) = 0.92, p = 0.37; t(16) = 1.7, p = 0.1; and t(17) = 0.37, p = 0.72 for AM251, BAPTA, and PDP-EA, respectively). C, Representative traces and summarized bar graph show that genetic knock-out of RGS9–2 did not significantly change PPR in D1-MSNs (aCSF; t(33) = 1.6, p = 0.13). Application of AM251, BAPTA, or PDP-EA did not have a significant effect on PPR in D1-MSNs (t(17) = 0.35, p = 0.73; t(14) = 0.67, p = 0.51; and t(13) = 1.2, p = 0.24 for AM251, BAPTA, and PDP-EA, respectively, compared between genotypes). Bar represents number of animals and cells used in each group. **p < 0.01, significantly different between groups.