Abstract
To examine how nutritional quality and resident gut bacteria interplay in improving the fitness of an oligophagous fruit fly, Bactrocera minax, artificial sucrose diets and full diets (sucrose, tryptone and yeast extract) were fed to flies with and without antibiotic supplementation. Furthermore, Klebsiella oxytoca and Citrobacter freundii were supplemented to sucrose-only diets. Flies were maintained in the laboratory and the fitness parameters, male and female longevity, number of copulations and female fecundity, were recorded. Full diet without bacterial depletion significantly increased fecundity and copulation. In the absence of gut bacteria, flies fed with full diets had significantly decreased mean fecundity and copulation rate. Flies that were fed with sucrose diet had a very low copulation rate and produced no eggs. Diet type and the presence of bacteria did not have any effect on the average longevity of male and female flies. Bacterial supplementation in sucrose diets did not improve any of the measured parameters. The results demonstrate that gut bacteria interact with diet to influence mating and reproduction in B. minax. Symbiotic bacteria significantly and positively impact reproduction in B. minax; however, their impact can only be fully realized when the flies are fed with a nutritionally complete diet.
Keywords: Tephritidae, diet quality, symbiotic bacteria, Dacinae
1. Introduction
Many insect species are able to survive and reproduce despite feeding on what are nutritionally poor diets. For example, aphids live on plant sap which is of low nutritional quality [1], while termites live on a non-degradable cellulose diet [2]. That these organisms, and numerous other examples, are nutritionally successful is considered to be the result of diet modification by symbiotic bacteria that these insects harbour [3].
The relationship between insects and their symbiotic bacteria dates back to the most primitive insects [4] and has had major impacts on insect evolution [3,5,6]. Resident bacteria have been isolated from the digestive tracts of many different insects, and the important roles they play in insect nutrition were widely accepted [7–9]. In some cases, the symbiont and its host insect have developed a very intimate relationship, where they depend primarily on each other for nutrition [1]. In other cases, the role symbiotic bacteria play in nutrition is less apparent, except when the host insect is nutritionally stressed. For example, Hamiltonella sp. infested white flies grew better than uninfected flies when reared with low nitrogen diets. However, no significant differences were observed when both groups were reared on a standard diet [10].
Fruit flies of the family Tephritidae (Insecta: Diptera) have close relationships with symbiotic bacteria [11,12], and gut bacteria form an essential part of the nutritional ecology of fruit flies [13,14]. Commonly, gut microbial fauna synthesize amino acids which do not otherwise occur in the diet, and this can lead to increased protein synthesis and female fecundity. Such results have been reported for the olive fruit fly, Bactrocera oleae (Rossi) [13,15], the apple maggot fly, Rhagoletis pomonella (Walsh) [16] and the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) [17,18].
While there are numerous papers supporting the link between bacteria and fruit fly nutrition, the field is not without ambiguity, or the need for further species-specific research. For example, Drew et al. [19], determined that the fecundity of Queensland fruit fly, Bactrocera tryoni (Froggatt), did not differ when fed a diet of bacteria, sugar and water versus a diet of brewer's yeast, sugar and water. This author, therefore, concluded that bacteria act as a natural food source for these fruit flies. However, Meats et al. [20] did not record any differential fecundity effects of bacteria feeding to B. tryoni. Such mixed results reinforce a point that for many fruit flies it is still unclear if bacteria act as a direct dietary source, act as modifiers of other diet, or play one or both roles but with an impact only being apparent if the host insect has, in some way, a dietary stress.
Bactrocera minax (Enderlein), known for breeding only on a citrus host plant, causes serious economic damage in southern/central China, Bhutan and neighbouring regions [21,22]. In support of pest management, significant research effort is currently being applied to understanding B. minax biology and ecology, including its bacterial relationships [23–25]. A previous study [26] on the impact of isolated bacterial strains on the fitness of the B. minax fruit fly had complex results which varied depending on the bacterial strain and the fitness parameters measured. In general, the incorporation of Pseudomonas dispersa, Klebsiella pneumonia and Citrobacter braakii into full diets decreased the longevity of male and female flies and improved female fecundity. However, only C. braakii supplementation significantly increased the number of observed matings. Though this study informs us that bacteria can influence fitness parameters of B. minax, it still remains unclear if these impacts are a result of the supplemented bacteria acting as additional dietary components, whether the bacteria modify the quality of the base diet, or if it is due to an interaction of both effects. It should be noted here that members of the genera Citrobacter and Klebsiella dominate the gut microbial fauna of many tephritid species including C. capitata [27], the oriental fruit fly, Bactrocera dorsalis (Hendel) [28] and B. minax [24].
To determine the mechanism(s) of how variable diet and gut bacteria interact to modify fitness of B. minax, we carried out two types of bacterial modification (antibiotic knock-down and bacterial supplementation) with two diet types (sucrose-only and sucrose + protein (= ‘full’) diets) and fed to adult flies. Our recorded fitness measures were, as for [26], longevity, fecundity and mating number. We hypothesized that the presence of gut bacteria will supplement the nutrition provided by both the sugar and ‘full’ diets, resulting in an overall improvement in fitness; while the absence of the gut bacteria would negatively impact the fitness parameters.
2. Material and methods
2.1. Insects and rearing conditions
All insects were collected from infested fruit from the Yichang district (30.69° N, 111.29° E), Hubei Province, China, and returned to laboratory at Huazhong Agricultural University, Wuhan. Third-instar larvae were allowed to pupate into sterilized soil in the laboratory, with subsequent adult emergence into sterilized mesh cages supplied with sugar and water. The laboratory conditions were as follows: temperature 27 ± 1°C, RH 70%, 12 L: 12 D. A total of 20 (10 males and 10 females) flies were held in each cage (45 × 30 × 30 cm) and each treatment was replicated three times. Replication number was restricted by the need to rear adults from naturally infested fruit, as it is not currently possible to laboratory culture this fly over multiple generations as it is univoltine with an obligate six-month diapause [21].
2.2. Experiment 1, interaction of diet and antibiotic treatments
Adult flies were used three days after emergence. Experimental flies were fed with double-distilled water-based sucrose or ‘full’ diets, with or without antibiotics. Flies fed with dissolved sucrose diets had no source of protein. By contrast, the ‘full’ diet contained sucrose, trypton and yeast extract (Beijing Shuangxuan microbial culture medium product factory, Beijing, China), which supplied flies with amino acids, minerals and carbohydrates [29]: diet details are provided in table 1. When antibiotics (10 µg ml−1 ciprofloxacin and 200 µg ml−1 piperacillin) were added to diet, it was first diluted in sterilized, double-distilled (DD) water and then filter-sterilized. The antibiotics used in this experiment have previously been used to suppress the gut bacteria in C. capitata and B. oleae [17,30–32]; however, antimicrobial susceptibility tests were carried out to confirm that bacteria strains isolated from the gut of the B. minax used had not developed resistance against these antibiotics (data available upon request from the senior author). After preparation, experimental diets were refrigerated at −20°C and used within one month. Diets were pipetted into Petri dishes containing a filter paper and offered and changed on a daily basis.
Table 1.
Composition of four experimental diets fed to B. minax adults (values in µg ml−1 of double-distilled water). The yeast extract used is from Beijing Shuangxuan microbial culture medium product factory, Beijing, China.
| sucrose | sucrose + antibiotics | full diet | full diet + antibiotics | |
|---|---|---|---|---|
| sucrose | 200 000 | 200 000 | 200 000 | 200 000 |
| yeast extract | 40 000 | 40 000 | ||
| trypton | 60 000 | 60 000 | ||
| antibiotics | ||||
| gentamicin | 10 | 10 | ||
| piperacillin | 200 | 200 |
2.3. Experiment 2, bacterial supplementation of sugar-only diets
In a separate experiment, we further investigated if specific bacteria isolates could improve on the low nutritional quality of sugar diets. Newly emerged flies were fed with antibiotics and sucrose diets for 4 days, then a concentration of 108 CFU ml−1 Klebsiella oxytoca and Citrobacter freundii previously isolated from the gut of B. minax were separately supplemented in the sucrose diet. Isolation and identification of these bacteria strains was carried out as reported by [26]. These sequences have been submitted to the NCBI GenBank under the reference number KF145191 and KF145194. Other than the diet, all other aspects of this trial were identical to the first.
2.4. Population fitness assessment
2.4.1. Longevity
The number and sex of dead insects per cage were determined each day until all flies died. The value used in the analysis was the mean longevity in days for each sex and each cage.
2.4.2. Mating
Bactrocera minax mates on the host plant, with peak mating during the middle of the day [25,33]. To mimic this, experimental cages were provided with a small potted valencia orange plant (Citrus c. * sinesis (L)). Ten sexually matured virgin males and females were kept within a cage and the number of mating pairs was recorded from 11.00 to 18.00 each day for 15 consecutive days for each of the three replicate treatment groups. The summed number of pairs over 15 days was the replicate data used for analysis.
2.4.3. Fecundity
Orange fruits were placed in experimental cages as oviposition substrates. Fruits were changed every three days and the number of eggs per fruit was counted following fruit dissection. The average fecundity per female was calculated by dividing the total number of eggs collected from each cage at the end of the experiment by the average number of females present in each cage during the fecundity period.
2.5. Statistical analysis
For experiment 1, a two-way ANOVA was performed to test the effects of diet type and antibiotics on the longevity (male and female), and copulation number. The independent variables were diet (sugar diet versus full diet) and antibiotic treatment (present versus absent). The dependent variables were mean male and female longevity per replicate and total number of matings per replicate. For fecundity, flies on the sugar-only diet, with or without antibiotics, produced no eggs, so the effect of diet was not statistically tested. The effect of antibiotic treatment on egg production of flies on the full diet (Expt 1), and bacterial supplementation in sugar diets (Expt 2) were tested using one-way ANOVA. Levene's test was used to assess homogeneity of all the datasets and only the effects of supplemented bacteria on male longevity required log transformations. Multiple comparisons between treatments were based on Tukey's post hoc tests. A p-value ≤ 0.05 was taken as significant. All datasets were analysed using SPSS 16.0 statistical software.
3. Results
3.1. Longevity
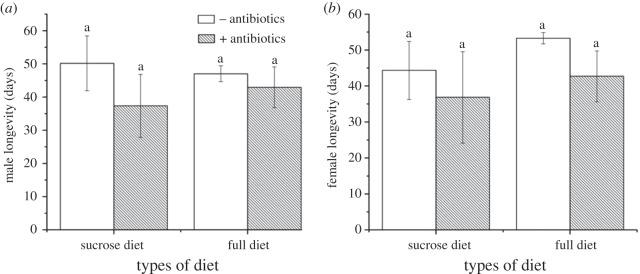
Diet type, antibiotic treatment and their interaction had no significant effect on the mean longevity of male or female B. minax (figure 1, table 2). Similarly, bacterial supplementation of a sucrose-only diet did not have any effect on the longevity of male and female flies (table 3, F2,6 = 1.648, p = 0.269 and F2,6 = 3.162, p = 0.115, respectively).
Figure 1.
The effects of diet type and antibiotic treatment (10 µg ml−1 ciprofloxacin and 200 µg ml−1 piperacillin) on the mean (s.d.) longevity in days of male (a) and female (b) Bactrocera minax. Unshaded bars are diets with no antibiotics added; shaded bars are diets with antibiotics added. Columns with the same letters are not significantly different after comparison with Tukey's test at p ≤ 0.05.
Table 2.
Effects of diet type, antibiotic treatment and their interaction on the longevity of B. minax. Separate two-way ANOVAs were run for males and females.
| male longevity |
female longevity |
|||
|---|---|---|---|---|
| source of variation | F1, 8 | p-value | F1,8 | p-value |
| diet | 0.91 | 0.77 | 2.37 | 0.16 |
| antibiotic treatment | 4.22 | 0.074 | 3.51 | 0.10 |
| diet × antibiotic treatment | 1.13 | 0.32 | 0.10 | 0.76 |
Table 3.
The effect of diet supplementation with bacteria and antibiotics (10 µg ml−1 ciprofloxacin and 200 µg ml−1 piperacillin) on different fitness parameters of B. minax. SA, sucrose + antibiotics; SAKO, SA + K. oxytoca; SACF, SA + C. freundii. Rows with the same letters are not significantly different at p ≤ 0.05.
| treatments |
|||
|---|---|---|---|
| SA | SAKO | SACF | |
| male longevity | 49.643 ± 0.41a | 40.767 ± 0.41a | 44.167 ± 0.41a |
| female longevity | 55.27 ± 3.61a | 48.43 ± 3.61a | 61.28 ± 3.61a |
| copulation | 0.67 ± 0.58a | 2.67 ± 0.58a | 1.67 ± 0.58a |
| fecundity | 0 | 0 | 0 |
3.2. Number of matings
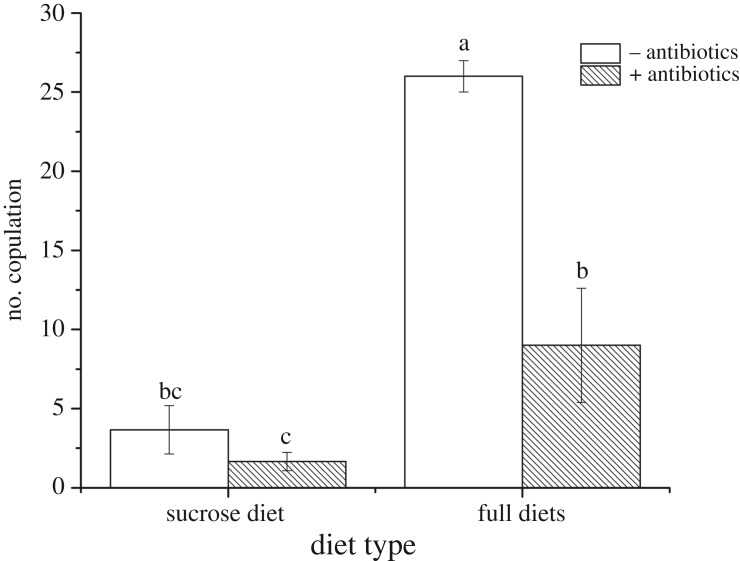
Flies fed sucrose diet achieved significantly fewer copulations than flies fed full diets (F1,8 = 158.42, p < 0.001). Similarly, flies fed with antibiotics achieved significantly fewer copulations than flies without antibiotic treatment (F1,8 = 64.980, p < 0.001) (figure 2). There was a significant interaction between diet and antibiotics (significant F1,8 = 168.75, p < 0.001). The number of copulations achieved by flies on full diets with antibiotics was significantly lower than the number of copulations achieved by flies on full diets without antibiotics. For the sucrose diet, addition of antibiotics to the diet did not significantly reduce (the already low) number of matings (figure 2). Bacterial supplementation to sucrose diets did not significantly alter the number of matings from the sucrose-only diets (table 3, F2.6 = 4.11, p = 0.125).
Figure 2.
Effect of diet quality and antibiotics (10 µg ml−1 ciprofloxacin and 200 µg ml−1 piperacillin) on the mean (±s.d.) number of B. minax copulations. Unshaded bars are diets with no antibiotics added, shaded bars are diets with antibiotics added. Columns with the same letters are not significantly different after comparison with Tukey's test at p ≤ 0.05.
3.3. Female fecundity
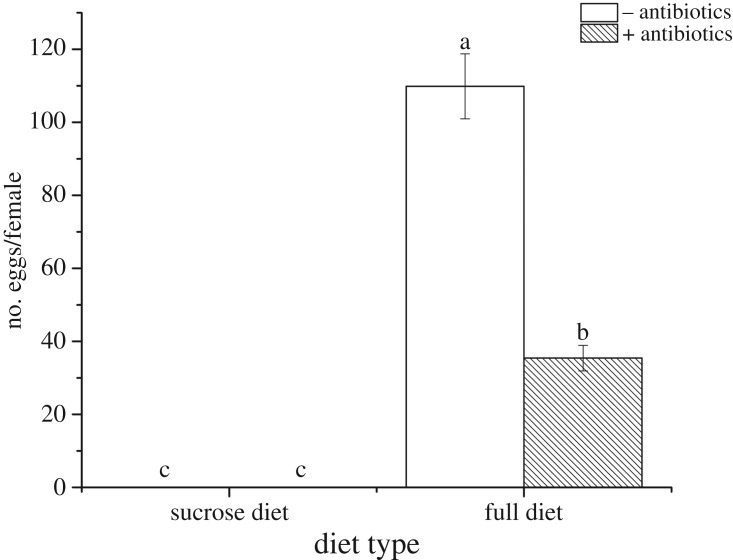
Diet type dramatically impacted egg production, with flies that fed on a sucrose-only diet, with or without antibiotics, laying no eggs. Similarly, females with bacterial supplementation to sucrose diets did not produce any eggs (table 3). For flies on the full diet, antibiotic treatment significantly reduced the number of eggs laid (F1,4 = 182.38, p < 0.0001) (figure 3).
Figure 3.
Effect of diet quality and antibiotic treatment on mean (±s.d.) number of eggs laid by B. minax. Unshaded bars are diets with no antibiotics added, shaded bars are diets with antibiotics added. Columns with the same letters are not significantly different after comparison Tukey's test at p ≤ 0.05.
4. Discussion
4.1. Summary
Although quantification of bacterial knock-down was not directly estimated after antibiotic treatment, its effect on fitness was seen in the results. The removal of gut bacteria through antibiotic treatment had no effects on the longevity of male and female B. minax (figure 1, table 2), but did reduce mating success and egg number. The effects of antibiotic treatments were only significant in the full diets, as the sucrose-only diet was apparently so poor that few matings were observed and no eggs at all were laid (figure 2), even after bacterial supplementation (table 3). Combined, these results strongly infer that bacteria alone are not an adequate source of nitrogen for B. minax, but the bacteria do mediate and improve the quality of diet when at least some alternative protein source is available.
4.2. Comparison of bacteria effects on fitness of B. minax versus studies in other tephritids
The gut of the B. minax fly is inhabited by a wide range of symbiotic bacteria [24] (herein referred to as resident bacteria) which enhanced fecundity and copulation rates of flies fed with full diets (data herein and [26]). Limited but similar results have been reported in other tephritids. In B. oleae, symbiotic bacteria have been reported to improve on the fecundity of flies fed with sucrose and non-essential amino acids [17] or bird droppings [13]. Similarly, Ben-Yosef et al. [30] reported that in the presence of resident bacteria, C. capitata fed with full diets had a reduced latency to mate.
While bacterial presence may improve dietary outcomes when amino acids/proteins are part of diet, this does not appear to be the case for carbohydrate diets only. The presence of resident bacteria did not improve any of the measured fitness parameters in sugar-only fed B. minax flies. Studies with B. oleae [13,17] and C. capitata have reported similar findings [30,31]. However, after antibiotics depletion of resident bacteria in C. capitata, flies that were fed with sugar diets laid eggs faster than those fed on full diets [30] and increased male longevity [31]. In Drosophila, axenic conditions do not lead to a significant increase in lifespan [34], while in the nematode Caenorhabditis elegans axenic conditions significantly increased lifespan [35]. From such studies, it is clear that the impact of resident bacteria on host fitness varies depending on the species.
When bacteria were supplemented in the diet of B. tryoni, no improvement on fitness was observed [20], a result identical to those was obtained for B. minax. However, in C. capitata, supplementations with Pseudomonas sp. resulted in a decrease in longevity [36] and supplementation with Enterobacteriacea resulted in an increase in mating competiveness [37] and longevity [36]. Similarly, when C. braaki and K. pneumonia were supplemented in full diet of B. minax, there was an increase in mating and fecundity [26]. The conflict between these studies again reinforces the likely unique nature of the bacterial/fruit fly interaction, which appears to vary depending on bacterial species, fly species, other dietary components and the fitness parameter being measured.
4.3. Bacteria effects as modified by protein-rich versus protein-poor diets
In many tephritid species, a protein diet is necessary for sexual maturation and oogenesis of the female fly [38,39]. In the absence of a protein diet, B. minax were not able to produce eggs, even when their diets were supplemented with bacteria (figure 3). However, when protein was present along with bacteria, there were positive fitness outcomes. Two hypotheses have been postulated to explain how diet and bacteria may work together. The first hypothesis that fruit fly can acquire protein and other nutrients by cultivating and digesting their gut bacteria [40,41], and the presence of additional protein enhances bacterial cultivation. With respect to this, it should be noted that the yeast extract and trypton used in our experiment supports bacterial growth in the laboratory. The second hypothesis proposes that fruit fly gut bacteria use amino acids already present in the diet as building blocks for essential amino acids, which are then used by the fly in their free form or integrated into bacterial protein after their secretion into the gut [13,17]. Because the presence of bacteria in a sucrose-only diet still did not allow the production of eggs by B. minax, we can infer that bacteria are not used directly by the fly as protein source, and so discount the first hypothesis. This leaves hypothesis 2 as the most parsimonious explanation: gut bacteria in B. minax alter the quality (i.e. type) of amino acid in the fly's diet and this accounts for the significant interaction between bacteria and diet quality.
Ethics
Bactrocera minax is indigenous in China. No permits were required for its collection and manipulation.
Data accessibility
The sequences of K. oxytoca and C. freundii used in this experiment have been submitted to the NCBI GenBank under the reference number KF145191 and KF145194, respectively.
Authors' contributions
C-Y.N. and J.H. designed the study, L.W., X-p.D. and A.A.A. prepared all the samples for the analysis and collected the data. A.A.A. and M.A. analysed the data, A.R.C. and A.A.A. interpreted the results and wrote the manuscript. All the authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by National Natural Science Foundation of China (31661143045 and 31371945), International Atomic Energy Agency (CRP nos. 17153 and 18269), Crop Disease and Insect Pest Monitoring and Control Program supported by Ministry of Agriculture of People's Republic of China (10162130108235049) and the Fundamental Research Funds for the Central Universities (2662015PY148).
References
- 1.Douglas A. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43, 17–37. ( 10.1146/annurev.ento.43.1.17) [DOI] [PubMed] [Google Scholar]
- 2.Tokuda G, Watanabe H, Matsumoto T, Noda H. 1997. Cellulose digestion in the wood-eating higher termite, Nasutitermes takasagoensis (Shiraki): distribution of cellulases and properties of endo-β-1, 4-gIucanase. Zoolog. Sci. 14, 83–93. ( 10.2108/zsj.14.83) [DOI] [PubMed] [Google Scholar]
- 3.Bourtzis K, Miller TA (eds). 2003. Insect symbiosis. Boca Raton, FL: CRC Press. [Google Scholar]
- 4.Chen R, Wang Z, Chen J, Jiang L-Y, Qiao G-X. 2017. Insect-bacteria parallel evolution in multiple-co-obligate-aphid association: a case in Lachninae (Hemiptera: Aphididae). Sci. Rep. 7, 10204 ( 10.1038/s41598-017-10761-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margulis L, Fester R (eds). 1991. Symbiosis as a source of evolutionary innovation: speciation and morphogenesis. Cambridge, MA: MIT Press. [PubMed] [Google Scholar]
- 6.Margulis L, Sagan D (eds). 2008. Acquiring genomes: a theory of the origins of species. New York, NY: Basic Books. [Google Scholar]
- 7.Gündüz EA, Douglas A. 2009. Symbiotic bacteria enable insect to use a nutritionally inadequate diet. Proc. R. Soc. B 276, 987–991. ( 10.1098/rspb.2008.1476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas AE. 2015. Multiorganismal insects: diversity and function of resident microorganisms. Annu. Rev. Entomol. 60, 17–34. ( 10.1146/annurev-ento-010814-020822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engel P, Moran NA. 2013. The gut microbiota of insects–diversity in structure and function. FEMS Microbiol. Rev. 37, 699–735. ( 10.1111/1574-6976.12025) [DOI] [PubMed] [Google Scholar]
- 10.Su Q, Xie W, Wang S, Wu Q, Liu B, Fang Y, Xu B, Zhang Y. 2014. The endosymbiont Hamiltonella increases the growth rate of its host Bemisia tabaci during periods of nutritional stress. PLoS ONE 9, e89002 ( 10.1371/journal.pone.0089002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurkevitch E. 2011. Riding the Trojan horse: combating pest insects with their own symbionts. Microb. Biotechnol. 4, 620–627. ( 10.1111/j.1751-7915.2011.00249.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauzon CR. 2003. Symbiotic relationships of tephritids, vol. 1. Boca Raton, FL: CRC Press. [Google Scholar]
- 13.Ben-Yosef M, Pasternak Z, Jurkevitch E, Yuval B. 2014. Symbiotic bacteria enable olive flies (Bactrocera oleae) to exploit intractable sources of nitrogen. J. Evol. Biol. 27, 2695–2705. ( 10.1111/jeb.12527) [DOI] [PubMed] [Google Scholar]
- 14.Hagen KS. 1966. Dependence of the olive fly, Dacus oleae, larvae on symbiosis with Pseudomonas savastanoi for the utilization of olive. Nature 209, 423–424. ( 10.1038/209423a0) [DOI] [Google Scholar]
- 15.Tsiropoulos G. 1984. Amino-acid synthesis in adult Dacus oleae (Gmelin)(Diptera Tephritidae) determined with [U-14C] glucose. Arch. Int. Physiol. Bochim. 92, 313–316. ( 10.3109/13813458409071172) [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki S, Boush GM, Baerwald RJ. 1968. Amino acid synthesis by Pseudomonas melophthora, bacterial symbiote of Rhagoletis pomonella (Diptera). J. Insect. Physiol. 14, 513–518. ( 10.1016/0022-1910(68)90066-8) [DOI] [PubMed] [Google Scholar]
- 17.Ben-Yosef M, Aharon Y, Jurkevitch E, Yuval B. 2010. Give us the tools and we will do the job: symbiotic bacteria affect olive fly fitness in a diet-dependent fashion. Proc. R. Soc. B 277, 1545–1552. ( 10.1098/rspb.2009.2102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behar A, Yuval B, Jurkevitch E. 2005. Enterobacteria-mediated nitrogen fixation in natural populations of the fruit fly Ceratitis capitata. Mol. Ecol. 14, 2637–2643. ( 10.1111/j.1365-294X.2005.02615.x) [DOI] [PubMed] [Google Scholar]
- 19.Drew R, Courtice A, Teakle D. 1983. Bacteria as a natural source of food for adult fruit flies (Diptera: Tephritidae). Oecologia 60, 279–284. ( 10.1007/BF00376839) [DOI] [PubMed] [Google Scholar]
- 20.Meats A, Streamer K, Gilchrist A. 2009. Bacteria as food had no effect on fecundity during domestication of the fruit fly, Bactrocera tryoni. J. Appl. Entomol. 133, 633–639. ( 10.1111/j.1439-0418.2009.01420.x) [DOI] [Google Scholar]
- 21.Dong Y-C, Wang Z-J, Clarke AR, Pereira R, Desneux N, Niu C-Y. 2013. Pupal diapause development and termination is driven by low temperature chilling in Bactrocera minax. J. Pest Sci. 86, 429–436. ( 10.1007/s10340-013-0493-y) [DOI] [Google Scholar]
- 22.Dorji C, Clarke AR, Drew RAI, Fletcher BS, Loday P, Mahat K, Raghu S, Romig MC. 2006. Seasonal phenology of Bactrocera minax (Diptera: Tephritidae) in western Bhutan. Bull. Entomol. Res. 96, 531–538. [PubMed] [Google Scholar]
- 23.Liu H, Jiang G, Zhang Y, Chen F, Li X, Yue J, Ran C, Zhao Z. 2015. Effect of six insecticides on three populations of Bactrocera (Tetradacus) minax (Diptera: Tephritidae). Curr. Pharm. Biotechnol. 16, 77–83. ( 10.2174/138920101601150105105751) [DOI] [PubMed] [Google Scholar]
- 24.Wang A, Yao Z, Zheng W, Zhang H. 2014. Bacterial communities in the gut and reproductive organs of Bactrocera minax (Diptera: Tephritidae) based on 454 pyrosequencing. PLoS ONE 9, e106988 ( 10.1371/journal.pone.0106988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X-L, Zhang R-J. 2009. Review on biology, ecology and control of Bactrocera (Tetradacus) minax Enderlein. J. Environ. Entomol. 31, 73–79. [Google Scholar]
- 26.Rashid MA, Andongma AA, Dong Y-C, Ren X-M, Niu C-Y. 2018. Effect of gut bacteria on fitness of the Chinese citrus fly, Bactrocera minax (Diptera: Tephritidae). Symbiosis 1–7. ( 10.1007/s13199-018-0537-4) [DOI] [Google Scholar]
- 27.Aharon Y, Pasternak Z, Ben-Yosef M, Behar A, Lauzon C, Yuval B, Jurkevitch E. 2013. Phylogenetic, metabolic, and taxonomic diversities shape Mediterranean fruit fly microbiotas during ontogeny. Appl. Environ. Microbiol. 79, 303–313. ( 10.1128/AEM.02761-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andongma AA, Wan L, Dong Y-C, Desneux N, White JA, Niu C-Y. 2015. Pyrosequencing reveals a shift in symbiotic bacteria populations across life stages of Bactrocera dorsalis. Sci. Rep. 5, 9470 ( 10.1038/srep09470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neto S, et al. 2012. Mass-rearing of Mediterranean fruit fly using low-cost yeast products produced in Brazil. Sci. Agricola 69, 364–369. ( 10.1590/S0103-90162012000600004) [DOI] [Google Scholar]
- 30.Ben-Yosef M, Jurkevitch E, Yuval B. 2008. Effect of bacteria on nutritional status and reproductive success of the Mediterranean fruit fly Ceratitis capitata. Physiol. Entomol. 33, 145–154. ( 10.1111/j.1365-3032.2008.00617.x) [DOI] [Google Scholar]
- 31.Ben-Yosef M, Behar A, Jurkevitch E, Yuval B. 2008. Bacteria–diet interactions affect longevity in the medfly–Ceratitis capitata. J. Appl. Entomol. 132, 690–694. ( 10.1111/j.1439-0418.2008.01330.x) [DOI] [Google Scholar]
- 32.Ben-Yosef M, Pasternak Z, Jurkevitch E, Yuval B. 2015. Symbiotic bacteria enable olive fly larvae to overcome host defences. R. Soc. open sci. 2, 150170 ( 10.1098/rsos.150170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong Y, Wan L, Pereira R, Desneux N, Niu C. 2014. Feeding and mating behaviour of Chinese citrus fly Bactrocera minax (Diptera, Tephritidae) in the field. J. Pest Sci. 87, 647–657. ( 10.1007/s10340-014-0605-3) [DOI] [Google Scholar]
- 34.Ren C, Webster P, Finkel SE, Tower J. 2007. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 6, 144–152. ( 10.1016/j.cmet.2007.06.006) [DOI] [PubMed] [Google Scholar]
- 35.Houthoofd K, Braeckman BP, Lenaerts I, Brys K, De Vreese A, Van Eygen S, Vanfleteren JR. 2002. Axenic growth up-regulates mass-specific metabolic rate, stress resistance, and extends life span in Caenorhabditis elegans. Exp. Gerontol. 37, 1371–1378. ( 10.1016/S0531-5565(02)00173-0) [DOI] [PubMed] [Google Scholar]
- 36.Behar A, Yuval B, Jurkevitch E. 2008. Gut bacterial communities in the Mediterranean fruit fly (Ceratitis capitata) and their impact on host longevity. J. Insect. Physiol. 54, 1377–1383. ( 10.1016/j.jinsphys.2008.07.011) [DOI] [PubMed] [Google Scholar]
- 37.Ben Ami E, Yuval B, Jurkevitch E. 2010. Manipulation of the microbiota of mass-reared Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) improves sterile male sexual performance. Int. Soc. Microb. Ecol. J. 4, 28–37. [DOI] [PubMed] [Google Scholar]
- 38.Drew R, Yuval B (eds). 1999. The evolution of fruit fly feeding behavior. Boca Raton, FL: CRC Press. [Google Scholar]
- 39.Meats A, Leighton S. 2004. Protein consumption by mated, unmated, sterile and fertile adults of the Queensland fruit fly, Bactrocera tryoni and its relation to egg production. Physiol. Entomol. 29, 176–182. ( 10.1111/j.1365-3032.2004.00383.x) [DOI] [Google Scholar]
- 40.Panizzi AR, Parra JR (eds). 2012. Insect bioecology and nutrition for integrated pest management. Boca Raton, FL: CRC Press. [Google Scholar]
- 41.Lemos F, Terra WR. 1991. Digestion of bacteria and the role of midgut lysozyme in some insect larvae. Comp. Biochem. Physiol. B 100, 265–268. ( 10.1016/0305-0491(91)90372-K) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequences of K. oxytoca and C. freundii used in this experiment have been submitted to the NCBI GenBank under the reference number KF145191 and KF145194, respectively.