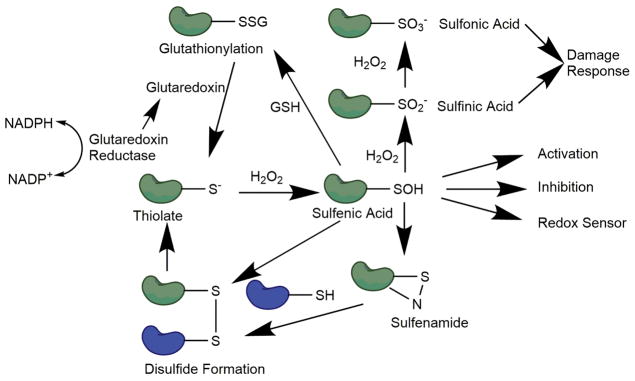
FIG. 1. General redox cycle of protein thiols.
In the presence of H2O2, thiols can be oxidized to sulfenic acids, which may elicit a physiological response. Sulfenic acids can then form disulfide bonds, which can be reduced to free thiols. Sulfenic acids can also be further oxidized to sulfinic or sulfonic acids, which can trigger cellular damage responses.